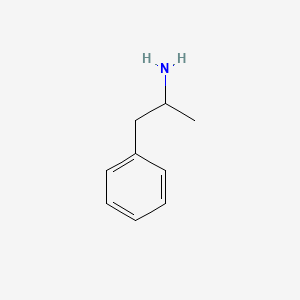
D0007 | Amphetamine
N
N06BA01 Amfetamine
[N06BA] Centrally acting sympathomimetics
[N06B] PSYCHOSTIMULANTS, AGENTS USED FOR ADHD AND NOOTROPICS
[N06] PSYCHOANALEPTICS
[N] Nervous system
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Danger |
Aggregated GHS information provided by 40 companies from 2 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H226 (95%): Flammable liquid and vapor [Warning Flammable liquids] H300 (100%): Fatal if swallowed [Danger Acute toxicity, oral] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P210, P233, P240, P241, P242, P243, P264, P270, P280, P301+P310, P303+P361+P353, P321, P330, P370+P378, P403+P235, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
 |
Danger |
The GHS information provided by 1 company from 1 notification to the ECHA C&L Inventory. H301 (100%): Toxic if swallowed [Danger Acute toxicity, oral] |
P264, P270, P301+P310, P321, P330, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
   |
Danger |
H225: Highly Flammable liquid and vapor [Danger Flammable liquids] H301: Toxic if swallowed [Danger Acute toxicity, oral] H311: Toxic in contact with skin [Danger Acute toxicity, dermal] H331: Toxic if inhaled [Danger Acute toxicity, inhalation] H370: Causes damage to organs [Danger Specific target organ toxicity, single exposure] |
P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P301+P310, P302+P352, P303+P361+P353, P304+P340, P307+P311, P311, P312, P321, P322, P330, P361, P363, P370+P378, P403+P233, P403+P235, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 15mg/kg (15mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 146, Pg. 392, 1963. | |
| mouse | LD50 | intraperitoneal | 4400ug/kg (4.4mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 161, Pg. 206, 1966. | |
| guinea pig | LDLo | parenteral | 20mg/kg (20mg/kg) | Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales. Vol. 128, Pg. 680, 1938. | |
| rat | LD50 | oral | 302mg/kg (302mg/kg) | Toxicology and Applied Pharmacology. Vol. 1, Pg. 42, 1959. | |
| rat | LD50 | subcutaneous | 180mg/kg (180mg/kg) | behavioral: excitement | Journal of Pharmacology and Experimental Therapeutics. Vol. 85, Pg. 119, 1945. |
| rat | LDLo | intraperitoneal | 23mg/kg (23mg/kg) | Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 195, Pg. 647, 1940. | |
| rabbit | LDLo | intravenous | 22mg/kg (22mg/kg) | behavioral: convulsions or effect on seizure threshold | Journal de Physiologie et de Pathologie Generale. Vol. 37, Pg. 597, 1939. |
| gerbil | LD50 | intraperitoneal | 17600ug/kg (17.6mg/kg) | Gerontology Vol. 23, Pg. 165, 1977. | |
| rabbit | LDLo | intravenous | 25mg/kg (25mg/kg) | "Structure et Activite Pharmacodyanmique des Medicaments du Systeme Nerveux Vegetatif," Bovet, D., and F. Bovet-Nitti, New York, S. Karger, 1948Vol. -, Pg. 149, 1948. | |
| rabbit | LDLo | subcutaneous | 20mg/kg (20mg/kg) | "Structure et Activite Pharmacodyanmique des Medicaments du Systeme Nerveux Vegetatif," Bovet, D., and F. Bovet-Nitti, New York, S. Karger, 1948Vol. -, Pg. 149, 1948. | |
| rat | LDLo | intraperitoneal | 20mg/kg (20mg/kg) | Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 195, Pg. 647, 1940. | |
| mammal (species unspecified) | LD50 | oral | 135mg/kg (135mg/kg) | Biochimie. Vol. 63, Pg. 495, 1981. | |
| rat | LD50 | subcutaneous | 200mg/kg (200mg/kg) | "Drug Dosages in Laboratory Animals - A Handbook," Rev. ed., Barnes, C.D., and L.G. Eltherington, Berkeley, Univ. of California Press, 1973Vol. -, Pg. 84, 1973. | |
| dog | LD50 | oral | 23mg/kg (23mg/kg) | Proceedings of the Society for Experimental Biology and Medicine. Vol. 118, Pg. 557, 1965. | |
| dog | LDLo | oral | 6400ug/kg (6.4mg/kg) | "Drug Dosages in Laboratory Animals - A Handbook," Rev. ed., Barnes, C.D., and L.G. Eltherington, Berkeley, Univ. of California Press, 1973Vol. -, Pg. 84, 1973. | |
| mouse | LD50 | intraperitoneal | 232mg/kg (232mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 158, Pg. 135, 1967. | |
| gerbil | LD50 | intraperitoneal | 17600ug/kg (17.6mg/kg) | behavioral: changes in motor activity (specific assay) | Gerontology Vol. 23, Pg. 165, 1977. |
| mouse | LD50 | unreported | 8800ug/kg (8.8mg/kg) | Acta Physiologica Academiae Scientiarum Hungaricae. Vol. 32, Pg. 377, 1967. | |
| mammal (species unspecified) | LD50 | intraperitoneal | 65mg/kg (65mg/kg) | Biochimie. Vol. 63, Pg. 495, 1981. | |
| guinea pig | LD50 | subcutaneous | 105mg/kg (105mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 137, Pg. 375, 1962. | |
| man | LDLo | unreported | 2206ug/kg (2.206mg/kg) | "Poisoning; Toxicology, Symptoms, Treatments," 2nd ed., Arena, J.M., Springfield, IL, C.C. Thomas, 1970Vol. 2, Pg. 73, 1970. | |
| child | TDLo | oral | 3600ug/kg/10D (3.6mg/kg) | behavioral: muscle contraction or spasticity) | American Journal of Psychiatry. Vol. 143, Pg. 1176, 1985. |
| domestic animals - goat/sheep | LDLo | subcutaneous | 15mg/kg (15mg/kg) | American Journal of the Medical Sciences. Vol. 198, Pg. 785, 1939. | |
| guinea pig | LDLo | intraperitoneal | 50mg/kg (50mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 100, Pg. 267, 1950. | |
| monkey | LDLo | subcutaneous | 5mg/kg (5mg/kg) | American Journal of the Medical Sciences. Vol. 198, Pg. 785, 1939. | |
| mouse | LD50 | intraperitoneal | 13mg/kg (13mg/kg) | Pharmaceutical Chemistry Journal Vol. 26, Pg. 430, 1992. | |
| mouse | LD50 | subcutaneous | 20mg/kg (20mg/kg) | behavioral: excitement | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 146, Pg. 392, 1963. |
| dog | LDLo | subcutaneous | 20mg/kg (20mg/kg) | "Structure et Activite Pharmacodyanmique des Medicaments du Systeme Nerveux Vegetatif," Bovet, D., and F. Bovet-Nitti, New York, S. Karger, 1948Vol. -, Pg. 149, 1948. | |
| mouse | LD50 | intravenous | > 100mg/kg (100mg/kg) | Journal of Medicinal Chemistry. Vol. 16, Pg. 1394, 1973. | |
| chicken | LD50 | intraperitoneal | 125mg/kg (125mg/kg) | behavioral: changes in motor activity (specific assay) | Toxicology and Applied Pharmacology. Vol. 2, Pg. 558, 1960. |
| rat | LD50 | intraperitoneal | 125mg/kg (125mg/kg) | behavioral: convulsions or effect on seizure threshold | Journal of Pharmacology and Experimental Therapeutics. Vol. 132, Pg. 97, 1961. |
| mouse | LD50 | oral | 40mg/kg (40mg/kg) | Toxicology and Applied Pharmacology. Vol. 41, Pg. 329, 1977. | |
| guinea pig | LDLo | subcutaneous | 20mg/kg (20mg/kg) | Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales. Vol. 128, Pg. 680, 1938. | |
| mammal (species unspecified) | LD50 | oral | 375mg/kg (375mg/kg) | Journal of Medicinal Chemistry. Vol. 8, Pg. 836, 1965. | |
| frog | LD50 | parenteral | 280mg/kg (280mg/kg) | behavioral: somnolence (general depressed activity) | Journal of Pharmacology and Experimental Therapeutics. Vol. 76, Pg. 375, 1942. |
| dog | LD50 | intravenous | 6mg/kg (6mg/kg) | Proceedings of the Society for Experimental Biology and Medicine. Vol. 118, Pg. 557, 1965. | |
| monkey | LDLo | oral | 32mg/kg (32mg/kg) | "Drug Dosages in Laboratory Animals - A Handbook," Rev. ed., Barnes, C.D., and L.G. Eltherington, Berkeley, Univ. of California Press, 1973Vol. -, Pg. 84, 1973. | |
| human | TDLo | oral | 41mg/kg (41mg/kg) | Klinische Wochenscrift. Vol. 17, Pg. 1580, 1938. | |
| guinea pig | LDLo | oral | 200mg/kg (200mg/kg) | Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales. Vol. 128, Pg. 680, 1938. | |
| rat | LD50 | subcutaneous | 160mg/kg (160mg/kg) | Annals of Internal Medicine. Vol. 10, Pg. 1874, 1937. | |
| monkey | LDLo | subcutaneous | 20mg/kg (20mg/kg) | "Structure et Activite Pharmacodyanmique des Medicaments du Systeme Nerveux Vegetatif," Bovet, D., and F. Bovet-Nitti, New York, S. Karger, 1948Vol. -, Pg. 149, 1948. | |
| man | TDLo | oral | 42mg/kg/25W-I (42mg/kg) | behavioral: toxic psychosis | Biological Psychiatry. Vol. 20, Pg. 1332, 1985. |
| rat | LD50 | oral | 38mg/kg (38mg/kg) | Toxicology and Applied Pharmacology. Vol. 18, Pg. 185, 1971. | |
| mouse | LD50 | oral | 24mg/kg (24mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 13, Pg. 711, 1963. | |
| chicken | LD50 | intravenous | 70mg/kg (70mg/kg) | behavioral: changes in motor activity (specific assay) | Toxicology and Applied Pharmacology. Vol. 2, Pg. 558, 1960. |
| mouse | LD50 | intravenous | 25mg/kg (25mg/kg) | Journal of Medicinal Chemistry. Vol. 15, Pg. 410, 1972. | |
| mouse | LD50 | intravenous | 31800ug/kg (31.8mg/kg) | Toxicology and Applied Pharmacology. Vol. 2, Pg. 558, 1960. | |
| mouse | LDLo | intraperitoneal | 52mg/kg (52mg/kg) | behavioral: ataxia | Journal of Pharmacology and Experimental Therapeutics. Vol. 127, Pg. 55, 1959. |
| mouse | LD50 | subcutaneous | 15mg/kg (15mg/kg) | Federation Proceedings, Federation of American Societies for Experimental Biology. Vol. 4, Pg. 139, 1945. | |
| rat | LDLo | subcutaneous | 160mg/kg (160mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 71, Pg. 62, 1941. | |
| mouse | LD50 | oral | 266mg/kg (266mg/kg) | Toxicology and Applied Pharmacology. Vol. 1, Pg. 42, 1959. | |
| mouse | LD50 | subcutaneous | 7mg/kg (7mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 170, Pg. 428, 1967. | |
| rat | LD50 | oral | 55mg/kg (55mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 13, Pg. 711, 1963. | |
| rat | LDLo | intravenous | 20mg/kg (20mg/kg) | "Structure et Activite Pharmacodyanmique des Medicaments du Systeme Nerveux Vegetatif," Bovet, D., and F. Bovet-Nitti, New York, S. Karger, 1948Vol. -, Pg. 101, 1948. | |
| mouse | LD50 | intraperitoneal | 5500ug/kg (5.5mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 161, Pg. 206, 1966. | |
| mouse | LD50 | oral | 21mg/kg (21mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 23, Pg. 810, 1973. | |
| rat | LD50 | oral | 30mg/kg (30mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 23, Pg. 810, 1973. | |
| (+)-.alpha.-Methylphenethylamine | (+)-.alpha.-Methylphenylethylamine | (+-)-Benzedrine |
| (+-)-alpha-Methylbenzeneethanamine | (+-)-alpha-Methylphenethylamine | (+-)-alpha-Methylphenylethylamine |
| (+/-)-Amphetamine solution, 1.0 mg/mL in methanol, ampule of 1 mL, certified reference material | (+/-)-Amphetamine solution, 100 mug/mL in methanol, ampule of 1 mL, certified reference material | (+/-)-Benzedrine |
| (+/-)-Desoxynorephedrine | (+/-)-beta-Phenylisopropylamine | (.+/-.)-.alpha.-Methylphenethylamine |
| (.+/-.)-.alpha.-Methylphenylethylamine | (.+/-.)-.beta.-Phenylisopropylamine | (.+/-.)-Benzedrine |
| (.+/-.)-Desoxynorephedrine | (Phenylisopropyl)amine | (S)-(+)-.beta.-Phenylisopropylamine |
| (S)-.alpha.-Phenylethylamine | (plusmn)-amphetamine | .alpha.-Methylbenzeneethanamine |
| .alpha.-Methylbenzeneethaneamine | .alpha.-Methylphenethylamine | .alpha.-Methylphenethylamine, d-form |
| .alpha.-Methylphenylethylamine | .beta.-Aminopropylbenzene | .beta.-Phenylisopropylamin |
| .beta.-Phenylisopropylamine | 1-Methyl-2-phenylethylamine | 1-Phenyl-2-amino-propan |
| 1-Phenyl-2-amino-propan [German] | 1-Phenyl-2-aminopropane | 1-Phenyl-2-aminopropane (VAN) |
| 1-Phenyl-2-propanamine | 1-Phenyl-2-propylamine | 1-phenylpropan-2-amine |
| 2-Amino-1-phenylpropane | 3-AMINO-1-PROPYLBENZENE | 3-Phenyl-2-propylamine |
| 3-phenylpropan-2-amine | 300-62-9 | 3797-EP2275420A1 |
| 3797-EP2295439A1 | 3797-EP2298734A2 | 3797-EP2298742A1 |
| 3797-EP2298779A1 | 3797-EP2305219A1 | 3797-EP2305648A1 |
| 3797-EP2305656A1 | 3797-EP2308867A2 | 3797-EP2308870A2 |
| 3797-EP2311801A1 | 3797-EP2311802A1 | 3797-EP2311803A1 |
| 3797-EP2314571A2 | 3797-EP2371814A1 | 41820-21-7 |
| 60-15-1 | AI3-02438 | ALPHA-METHYL-BENZENEETHANAMIDE (SEE ALSO DL-AMPHETAMINE SULFATE) |
| AMPHETAMINE | AMPHETAMINE (SEE ALSO: D-AMPHETAMINE (51-64-9) & AMPHETAMINE SULFATE (60-13-9)) | AMPHETAMINE, (D) |
| Actedron | Adderal | Adderall XR |
| Adipan | Adzenys ER | Allodene |
| Amfetamin (TN) | Amfetamina [INN-Spanish] | Amfetamina [Italian] |
| Amfetamine | Amfetamine (INN) | Amfetamine [INN:BAN] |
| Amfetaminum [INN-Latin] | Amphetamin | Amphetamine Sulfate (2:1) |
| Amphetamine mixture with Dextroamphetamine | Amphetamine resin complex | Amphetamine salts |
| Amphetamine, its salts, optical isomers, and salts of its optical isomers | Anfetamina [Spanish] | Anorexide |
| Anorexine | BDBM50005246 | Benzebar |
| Benzedrine | Benzeneethanamine, (S)- | Benzeneethanamine, .alpha.-methyl- |
| Benzeneethanamine, .alpha.-methyl-, (.+/-.)- | Benzeneethanamine, .alpha.-methyl-, (.alpha.S)- | Benzeneethanamine, .alpha.-methyl-, (S)- |
| Benzeneethanamine, alpha-methyl- | Benzeneethanamine, alpha-methyl-, (+-)- | Benzolone |
| C07514 | CHEBI:132233 | CHEBI:2679 |
| CHEMBL405 | D-1-Phenyl-2-aminopropan | D07445 |
| DB-047697 | DB00182 | DEA No. 1100 |
| DELCOBESE | DEXTROAMP SACCHARATE, AMP ASPARTATE, DEXTROAMP SULFATE AND AMP SULFATE | DL-.alpha.-Methylphenethylamine |
| DL-Amphetamine solution, 1 mg/mL in methanol, drug standard | DTXSID4022600 | Desoxynorephedrin |
| Desoxynorephedrine | Dexacaps | Dexedrine |
| DivK1c_000991 | Dyanavel XR | EINECS 200-458-3 |
| EINECS 206-096-2 | Elastonon | Euphodyn (Salt/Mix) |
| FT-0631912 | FT-0694835 | Fabedrine (Salt/Mix) |
| Fenamin (Salt/Mix) | Fenopromin | Fenylo-izopropylaminyl |
| Fenylo-izopropylaminyl [Polish] | Finam | GTPL4804 |
| HMS503G03 | HSDB 3287 | IDI1_000991 |
| Isoamycin | Isoamyne | Isomyn |
| KBio1_000991 | KWTSXDURSIMDCE-UHFFFAOYSA-N | L000864 |
| LS-1148 | LS-1428 | Levoamphetamine |
| MCULE-4193952437 | Mecodrin | Mydrial |
| NINDS_000991 | NSC 27159 | NSC-27159 |
| NSC-73713 | NSC27159 | NSC73713 |
| NT-0201 | Noclon (Salt/Mix) | Norephedrane |
| Norephedrine, deoxy- | Novydrine | Oktedrin |
| Oprea1_447423 | Oraldrina (Salt/Mix) | Ortedrine |
| Ortenal (Salt/Mix) | Percomon | Phenamine |
| Phenedrine | Phenethylamine, (+)- | Phenethylamine, .alpha.-methyl- |
| Phenethylamine, .alpha.-methyl-, (.+/-.)- | Phenethylamine, D-.alpha.-methyl- | Phenethylamine, alpha-methyl, (+-)- |
| Phenethylamine, alpha-methyl- | Phenethylamine, alpha-methyl-, (+-)- | Phenethylamine, d- |
| Profamina | Protioamphetamine | Psychedrine |
| Q179452 | S(+)-Amphetamine | SCHEMBL8858 |
| Simpatina | Stimulan (Salt/Mix) | Sympametin (Salt/Mix) |
| Thyramine | Vapedrine (Salt/Mix) | WLN: ZY1&1R |
| WLN: ZY1&1R -D | Zedrine (Salt/Mix) | alpha-Methylbenzeneethaneamine |
| alpha-Methylphenethylamine | alpha-Methylphenylethylamine | alpha-methyl phenethylamine |
| amfetaminum | amphetamine- | beta-Aminopropylbenzene |
| beta-Aminopropylbenzene (VAN) | beta-Phenylisopropylamin | beta-Phenylisopropylamin [German] |
| beta-Phenylisopropylamine | beta-phenyl-isopropylamine | component of Amodex |
| component of Biphetamine | d-.alpha.-Methylphenethylamine | d/l-Amphetamine hydrochloride |
| dl-1-Phenyl-2-aminopropane | dl-Amphetamine | dl-Benzedrine |
| dl-alpha-Methylphenethylamine | levo-Amphetamine | rac-(2R)-1-phenylpropan-2-amine |
| rac-Amphetamine | rac-Amphetamine 1.0 mg/ml in Methanol | racemic-Desoxynor-ephedrine |
| DrugBank Name | Amphetamine |
| DrugBank | DB00182 |
| CAS Number | 139-10-6, 156-34-3, 300-62-9, 3090-45-7, 41820-21-7, 51-62-7, 51-64-9, 60-13-9, 60-15-1 |
| PubChem Compound | 3007 |
| KEGG Compound ID | C07514 |
| KEGG Drug | D07445 |
| PubChem.Substance | 46506414 |
| ChEBI | 132233 |
| PharmGKB | PA448408 |
| ChemSpider | 13852819 |
| BindingDB | 50005246.0 |
| TTD | DAP001146 |
| Wikipedia | Amphetamine |
| DPD | 8300|8309|22635 |