Drug
D0020 | Cephaloglycin
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| RESPIRATION | inhibit | 197 | ||||||
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Danger |
Aggregated GHS information provided by 38 companies from 1 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H317 (100%): May cause an allergic skin reaction [Warning Sensitization, Skin] H334 (100%): May cause allergy or asthma symptoms or breathing difficulties if inhaled [Danger Sensitization, respiratory] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P261, P272, P280, P285, P302+P352, P304+P341, P321, P333+P313, P342+P311, P363, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | subcutaneous | 2800mg/kg (2800mg/kg) | kidney, ureter, and bladder: "changes in tubules (including acute renal failure, acute tubular necrosis)" | Chemotherapy Vol. 18, Pg. 22, 1970. |
| rat | LD50 | intraperitoneal | 1300mg/kg (1300mg/kg) | kidney, ureter, and bladder: "changes in tubules (including acute renal failure, acute tubular necrosis)" | Chemotherapy Vol. 18, Pg. 22, 1970. |
| rat | LD50 | oral | > 10gm/kg (10000mg/kg) | liver: other changes | Chemotherapy Vol. 18, Pg. 22, 1970. |
| mouse | LD50 | subcutaneous | 3700mg/kg (3700mg/kg) | kidney, ureter, and bladder: "changes in tubules (including acute renal failure, acute tubular necrosis)" | Chemotherapy Vol. 18, Pg. 22, 1970. |
| mouse | LD50 | intraperitoneal | 1030mg/kg (1030mg/kg) | kidney, ureter, and bladder: "changes in tubules (including acute renal failure, acute tubular necrosis)" | Chemotherapy Vol. 18, Pg. 22, 1970. |
| mouse | LD50 | oral | > 10gm/kg (10000mg/kg) | liver: other changes | Chemotherapy Vol. 18, Pg. 22, 1970. |
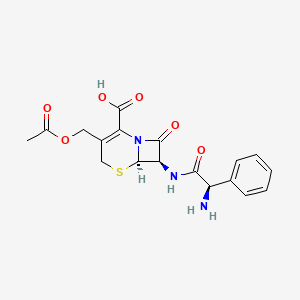
| (6R,7R)-3-(acetoxymethyl)-7-{[(2R)-2-amino-2-phenylacetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | (6R,7R)-3-[(acetyloxy)methyl]-7-[(2R)-2-amino-2-phenylacetamido]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | (6R,7R)-3-[(acetyloxy)methyl]-7-{[(2R)-2-amino-2-phenylacetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| 3-((Acetyloxy)methyl)-7-((aminophenylacetyl)amino)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid | 3-acetoxymethyl-7beta-[(2R)-2-amino-2-phenylacetamido]-3,4-didehydrocepham-4-carboxylic acid | 3577-01-3 |
| 5-Thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, 3-((acetyloxy)methyl)-7-((aminophenylacetyl)amino)-8-oxo-, (6R-(6alpha,7beta(R*)))- | 5-Thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, 7-(2-amino-2-phenylacetamido)-3-(hydroxymethyl)-8-oxo-, acetate (ester), D- | 7-(2-Amino-2-phenylacetamido)-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid acetate (ester) |
| 7-(2-Amino-2-phenylacetamido)-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)octane-2-carboxylic acid, acetate inner salt | 7-(2-D-alpha-Aminophenylacetamido)cephalosporanic acid | 7-(D-2-Amino-2-phenylacetamido)-3-acetoxymethyl-delta(sup 3)-cephem-4-carboxylic acid |
| 7-(D-2-Amino-2-phenylacetamido)-3-acetoxymethyl-delta(sup3)-cephem-4-carboxylic acid | 7-(D-alpha-Aminophenyl-acetamido)cephalosporanic acid | API0001931 |
| C13440 | C18H19N3O6S | CEG |
| CEPHALOGLYCIN | CHEBI:34613 | CHEMBL1200971 |
| Cefaloglicina | Cefaloglicina [INN-Spanish] | Cefaloglycin |
| Cefaloglycin (JAN) | Cefaloglycin [INN] | Cefaloglycine |
| Cefaloglycine [INN-French] | Cefaloglycinum | Cefaloglycinum [INN-Latin] |
| Cephaloglycin (anhydrous) | Cephaloglycin anhdyous | Cephaloglycin anhydrous |
| Cephaloglycine | Cephaoglycin acid | D-(-)-Cephaloglycin |
| D-Cephaloglycine | D01949 | DB00689 |
| DTXSID4022781 | EINECS 222-696-7 | Epitope ID:174844 |
| FUBBGQLTSCSAON-PBFPGSCMSA-N | HD2D469W6U | HSDB 3214 |
| Kafocin | Kefglycin | LS-149962 |
| Lilly 39435 | Q5057214 | SCHEMBL2947 |
| UNII-HD2D469W6U | ZINC3830503 |
| DrugBank Name | Cephaloglycin |
| DrugBank | DB00689 |
| CAS Number | 3577-01-03 00:00:00 |
| PubChem Compound | 19150 |
| KEGG Compound ID | C13440 |
| KEGG Drug | D01949 |
| PubChem.Substance | 46506850 |
| ChEBI | 34613 |
| PharmGKB | PA164781027 |
| ChemSpider | 18069 |
| TTD | DAP001158 |
| Wikipedia | Cephaloglycin |
1. Vuda et al. (2016)