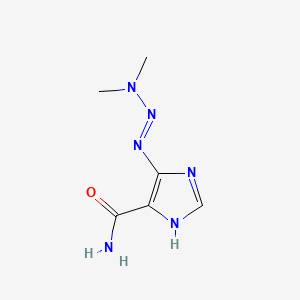
D0033 | Dacarbazine
L
L01AX04 Dacarbazine
[L01AX] Other alkylating agents
[L01A] ALKYLATING AGENTS
[L01] ANTINEOPLASTIC AGENTS
[L] Antineoplastic and immunomodulating agents
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Danger |
Aggregated GHS information provided by 42 companies from 5 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H302+H312+H332 (90.48%): Harmful if swallowed, in contact with skin or if inhaled [Warning Acute toxicity, oral acute toxicity, dermal acute toxicity, inhalation] H302 (92.86%): Harmful if swallowed [Warning Acute toxicity, oral] H312 (95.24%): Harmful in contact with skin [Warning Acute toxicity, dermal] H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H332 (95.24%): Harmful if inhaled [Warning Acute toxicity, inhalation] H335 (97.62%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure Respiratory tract irritation] H340 (95.24%): May cause genetic defects [Danger Germ cell mutagenicity] H350 (97.62%): May cause cancer [Danger Carcinogenicity] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P261, P264, P270, P271, P280, P281, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P308+P313, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P403+P233, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | oral | 2147mg/kg (2147mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 9, Pg. 3105, 1981. | |
| human | TDLo | intravenous | 3500ug/kg (3.5mg/kg) | Cancer Chemotherapy Reports, Part 1. Vol. 57, Pg. 83, 1973. | |
| rat | LD50 | intravenous | 411mg/kg (411mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 9, Pg. 3105, 1981. | |
| mouse | LD50 | oral | 2032mg/kg (2032mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 9, Pg. 3105, 1981. | |
| rat | LD50 | intraperitoneal | 350mg/kg (350mg/kg) | Archiv fuer Geschwulstforschung. Vol. 50, Pg. 306, 1980. | |
| hamster | LD10 | parenteral | 250mg/kg (250mg/kg) | Journal of Surgical Oncology. Vol. 15, Pg. 355, 1980. | |
| mouse | LD50 | intraperitoneal | 567mg/kg (567mg/kg) | Cancer Treatment Reports. Vol. 62, Pg. 721, 1978. | |
| mouse | LD50 | intravenous | 466mg/kg (466mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 9, Pg. 3105, 1981. | |
| Dacarbazine |
| DrugBank Name | Dacarbazine |
| DrugBank | DB00851 |
| CAS Number | 4342-03-4, 94361-71-4 |
| PubChem Compound | 135398738 |
| KEGG Compound ID | C06936 |
| KEGG Drug | D00288 |
| PubChem.Substance | 46507029 |
| ChEBI | 4305 |
| PharmGKB | PA449197 |
| ChemSpider | 10481959 |
| TTD | DAP000533 |
| Wikipedia | Dacarbazine |
| DPD | 2264 |