Drug
D0049 | Epirubicin
L
L01DB03 Epirubicin
[L01DB] Anthracyclines and related substances
[L01D] CYTOTOXIC ANTIBIOTICS AND RELATED SUBSTANCES
[L01] ANTINEOPLASTIC AGENTS
[L] Antineoplastic and immunomodulating agents
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Warning |
H302: Harmful if swallowed [Warning Acute toxicity, oral] |
P264, P270, P301+P312, P330, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | intraperitoneal | 16mg/kg (16mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 6, Pg. 1075, 1972. | |
| man | LDLo | unreported | 243ug/kg (0.243mg/kg) | Postgraduate Medical Journal. Vol. 68, Pg. 69, 1992. | |
| mouse | LD50 | intraperitoneal | 10700ug/kg (10.7mg/kg) | Huaxi Yike Daxue Xuebao. Journal of West China University of Medical Sciences. Vol. 20, Pg. 303, 1989. | |
| human | LDLo | intravenous | 400ug/kg (0.4mg/kg) | Cancer Chemotherapy Reports, Part 3. Vol. 3, Pg. 33, 1972. | |
| hamster | LD10 | parenteral | 3500ug/kg (3.5mg/kg) | Journal of Surgical Oncology. Vol. 15, Pg. 355, 1980. | |
| mouse | LD50 | unreported | 21900ug/kg (21.9mg/kg) | Biochemical Pharmacology. Vol. 38, Pg. 167, 1989. | |
| mouse | LD50 | intravenous | 10mg/kg (10mg/kg) | Journal of Antibiotics. Vol. 45, Pg. 1373, 1992. | |
| human | TDLo | intravenous | 380mg/kg/31W (380mg/kg) | Cancer Vol. 34, Pg. 518, 1974. | |
| rabbit | LDLo | intrapleural | 400ug/kg (0.4mg/kg) | Pharmacology and Toxicology Vol. 62, Pg. 84, 1988. | |
| rat | LD50 | unreported | 7mg/kg (7mg/kg) | Antibiotiki i Khimioterapiya. Antibiotics and Chemotherapy. Vol. 34, Pg. 216, 1989. | |
| rat | LD50 | intravenous | 10510ug/kg (10.51mg/kg) | Toxicology and Applied Pharmacology. Vol. 79, Pg. 412, 1985. | |
| mammal (species unspecified) | LD50 | intraperitoneal | 8500ug/kg (8.5mg/kg) | Antibiotiki. Vol. 29, Pg. 748, 1984. | |
| rabbit | LD50 | intravenous | 5mg/kg (5mg/kg) | Yiyao Gongye. Pharmaceutical Industry. Vol. 17, Pg. 72, 1986. | |
| human | TDLo | intravenous | 15mg/kg/D (15mg/kg) | Cancer Vol. 34, Pg. 518, 1974. | |
| mouse | LD50 | oral | 570mg/kg (570mg/kg) | Antibiotiki. Vol. 28, Pg. 298, 1983. | |
| dog | LD50 | intravenous | 2400ug/kg (2.4mg/kg) | Drug and Chemical Toxicology. Vol. 6, Pg. 21, 1983. | |
| mouse | LDLo | intratracheal | 2400ug/kg (2.4mg/kg) | Toxicology Letters. Vol. 30, Pg. 63, 1986. | |
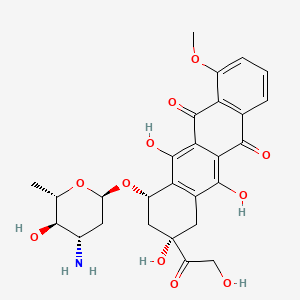
| (1S,3S)-3,5,12-trihydroxy-3-(hydroxyacetyl)-10-(methyloxy)-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-arabino-hexopyranoside | (1S,3S)-3,5,12-trihydroxy-3-(hydroxyacetyl)-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-arabino-hexopyranoside | (7S,9R)-7-[(2S,4S,5R,6S)-4-Amino-5-hydroxy-6-methyl-oxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione |
| (7S,9S)-7-[(2R,4S,5R,6S)-4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione | (7S,9S)-7-[(2R,4S,5R,6S)-4-azanyl-6-methyl-5-oxidanyl-oxan-2-yl]oxy-4-methoxy-6,9,11-tris(oxidanyl)-9-(2-oxidanylethanoyl)-8,10-dihydro-7H-tetracene-5,12-dione | (7S,9S)-7-[[(2R,4S,5R,6S)-4-amino-5-hydroxy-6-methyl-2-oxanyl]oxy]-6,9,11-trihydroxy-9-(2-hydroxy-1-oxoethyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione |
| (8S,10S)-10-{[(2R,4S,5R,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy}-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-5,7,8,9,10,12-hexahydrotetracene-5,12-dione | 10-((3-amino-2,3,6-trideoxy-beta-L-arabino-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-(8S-cis)-5,12-naphthacenedione | 15206-EP2269994A1 |
| 15206-EP2270008A1 | 15206-EP2270018A1 | 15206-EP2272827A1 |
| 15206-EP2275420A1 | 15206-EP2280012A2 | 15206-EP2281815A1 |
| 15206-EP2289892A1 | 15206-EP2292615A1 | 15206-EP2292617A1 |
| 15206-EP2295055A2 | 15206-EP2295416A2 | 15206-EP2295426A1 |
| 15206-EP2295427A1 | 15206-EP2298748A2 | 15206-EP2298764A1 |
| 15206-EP2298765A1 | 15206-EP2298768A1 | 15206-EP2298778A1 |
| 15206-EP2298780A1 | 15206-EP2301928A1 | 15206-EP2301933A1 |
| 15206-EP2305640A2 | 15206-EP2305642A2 | 15206-EP2305671A1 |
| 15206-EP2305689A1 | 15206-EP2308855A1 | 15206-EP2308861A1 |
| 15206-EP2311453A1 | 15206-EP2311808A1 | 15206-EP2311825A1 |
| 15206-EP2311827A1 | 15206-EP2311829A1 | 15206-EP2311840A1 |
| 15206-EP2311842A2 | 15206-EP2316832A1 | 15206-EP2316833A1 |
| 3Z8479ZZ5X | 4'-Epi-DXR | 4'-Epiadriamycin |
| 4'-Epidoxorubicin|||4'-Epiadriamycin|||(8S,10S)-10-(((2R,4S,5R,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione | 4'-epi-Doxorubicin | 4'-epidoxorubicin |
| 4-Epidoxorubicin | 5,12-Naphthacenedione, 10-((3-amino-2,3,6-trideoxy-alpha-L-arabino-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, (8S-cis)- | 5,12-Naphthacenedione, 10-((3-amino-2,3,6-trideoxy-beta-L-arabino-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, (8S-cis)- |
| 5,12-Naphthacenedione, 10-[(3-amino-2,3,6-trideoxy-.alpha.-L-arabino-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-, (8S,10S)- | 5,12-Naphthacenedione,10-[(3-amino-2,3,6-trideoxy-a-L-arabino-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, (8S,10S)- | 56420-45-2 |
| A12409 | A831042 | AB00698552-11 |
| AB00698552-13 | AB00698552-14 | AB00698552_15 |
| AB00698552_16 | AK326115 | AOJJSUZBOXZQNB-VTZDEGQISA-N |
| Acid, 8 | BDBM43839 | BRD-K04548931-003-16-5 |
| BRN 1445813 | C11230 | CCRIS 2261 |
| CHEBI:47898 | CHEMBL417 | CS-2824 |
| CTK8F9465 | D07901 | DB00445 |
| DTXSID0022987 | Doxorubicin-13CD3 (discontinued) | EPIRUBICIN(Hydrochloride form) |
| Ellence | Epi-DX | Epiadriamycin |
| Epidoxorubicin | Epirubicin | Epirubicin (INN) |
| Epirubicin [INN:BAN] | Epirubicina | Epirubicina [INN-Spanish] |
| Epirubicina [Spanish] | Epirubicine | Epirubicine [French] |
| Epirubicine [INN-French] | Epirubicinum | Epirubicinum [INN-Latin] |
| Epirubicinum [Latin] | FT-0630692 | Farmorubicin (TN) |
| HSDB 6962 | HY-13624 | IMI 28 |
| LS-187190 | LS-93992 | NCGC00263918-04 |
| NSC 256942 | NSC-256942 | NSC256942 |
| Pidorubicin | Pidorubicina | Pidorubicina [INN-Spanish] |
| Pidorubicine | Pidorubicine [INN-French] | Pidorubicinum |
| Pidorubicinum [INN-Latin] | Q425122 | Ridorubicin |
| SBI-0206890.P001 | SCHEMBL8582 | UNII-3Z8479ZZ5X |
| VA10821 | WP 697 | ZINC3938704 |
| DrugBank Name | Epirubicin |
| DrugBank | DB00445 |
| CAS Number | 23214-92-8, 56390-09-1, 56420-45-2 |
| PubChem Compound | 41867 |
| KEGG Compound ID | C11230 |
| KEGG Drug | D07901 |
| PubChem.Substance | 46507282 |
| ChEBI | 47898 |
| PharmGKB | PA449476 |
| ChemSpider | 38201 |
| BindingDB | 43839.0 |
| TTD | DAP000193 |
| Wikipedia | Epirubicin |
| DPD | 20259 |
1. Dykens et al. (2007)