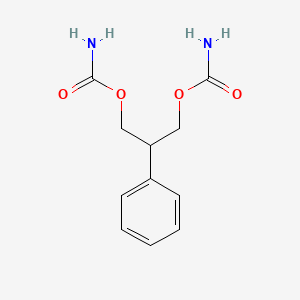
D0054 | Felbamate
N
N03AX10 Felbamate
[N03AX] Other antiepileptics
[N03A] ANTIEPILEPTICS
[N03] ANTIEPILEPTICS
[N] Nervous system
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Danger |
Aggregated GHS information provided by 27 companies from 2 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. Reported as not meeting GHS hazard criteria by 1 of 27 companies. For more detailed information, please visit ECHA C&L website Of the 1 notification(s) provided by 26 of 27 companies with hazard statement code(s): H317 (100%): May cause an allergic skin reaction [Warning Sensitization, Skin] H334 (100%): May cause allergy or asthma symptoms or breathing difficulties if inhaled [Danger Sensitization, respiratory] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P261, P272, P280, P285, P302+P352, P304+P341, P321, P333+P313, P342+P311, P363, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| (3-aminocarbonyloxy-2-phenyl-propyl) carbamate | (3-carbamoyloxy-1,1,3,3-tetradeuterio-2-phenylpropyl) carbamate | (3-carbamoyloxy-2-phenylpropyl) carbamate |
| 1,3-Propanediol, 2-phenyl-, dicarbamate | 2-?methyl-?4-?(4-?methyl-?1-?piperazinyl)-?10H-?thieno[2,?3-?b][1,?5]benzodiazepine | 2-Phenyl-1,3-propanediol dicarbamate |
| 2-Phenyl-1,3-propanedioldicarbamate | 2-Phenylpropane-1,3-diyl dicarbamate | 25451-15-4 |
| 3-(carbamoyloxy)-2-phenylpropyl carbamate | 4-06-00-05993 (Beilstein Handbook Reference) | 451F154 |
| A817858 | AB0011540 | AB00382985-18 |
| AB00382985_19 | AB1008593 | AC-8197 |
| ACN-054465 | ADD 03055 | ADD-03055 |
| AKOS015895100 | ANW-65602 | AOB5042 |
| API0002680 | AX8130043 | BCP27941 |
| BDBM50088430 | BIDD:GT0463 | BN0583 |
| BPBio1_001258 | BRD-K99107520-001-01-9 | BRD-K99107520-001-09-2 |
| BRD-K99107520-001-18-3 | BRN 3345236 | Biomol-NT_000203 |
| C-14192 | C07501 | C11H14N2O4 |
| CAS-25451-15-4 | CC-28364 | CCG-204614 |
| CHEBI:4995 | CHEMBL1094 | CS-2068 |
| Carbamic acid 3-carbamoyloxy-2-phenyl-propyl ester | Carbamic acid, 2-phenyltrimethylene ester | D00536 |
| DB-046702 | DB00949 | DSSTox_CID_3041 |
| DSSTox_GSID_23041 | DSSTox_RID_76847 | DTXSID9023041 |
| EINECS 247-001-4 | EN300-119542 | EU-0100524 |
| EX-A591 | F 0778 | FT-0630517 |
| Felbamate (USAN/INN) | Felbamate [USAN:INN] | Felbamate, United States Pharmacopeia (USP) Reference Standard |
| Felbamato | Felbamato [Spanish] | Felbamatum |
| Felbamatum [Latin] | Felbamyl | Felbatol |
| Felbatol (TN) | GTPL5473 | HMS2093P19 |
| HMS2234H06 | HMS3261J09 | HMS3266L12 |
| HMS3370I06 | HMS3411P21 | HMS3657I11 |
| HMS3675P21 | HMS3715D20 | HSDB 7525 |
| HY-B0184 | J90036 | K-6677 |
| KS-00001F6O | KS-1171 | LP00524 |
| LS-120706 | Lopac-F-0778 | Lopac0_000524 |
| MCULE-2661497722 | MFCD00865296 | MLS000028465 |
| MLS001077299 | NCGC00015429-01 | NCGC00015429-02 |
| NCGC00015429-03 | NCGC00015429-04 | NCGC00015429-05 |
| NCGC00015429-06 | NCGC00015429-07 | NCGC00015429-08 |
| NCGC00015429-09 | NCGC00015429-10 | NCGC00015429-11 |
| NCGC00015429-14 | NCGC00023092-02 | NCGC00023092-04 |
| NCGC00023092-05 | NCGC00023092-06 | NCGC00255275-01 |
| NCGC00261209-01 | NSC-759866 | NSC759866 |
| Opera_ID_1738 | Pharmakon1600-01505600 | Prestwick0_000919 |
| Q-100326 | Q421301 | SBI-0050507.P002 |
| SC-46002 | SCHEMBL34947 | SMR000058448 |
| SR-01000000089 | SR-01000000089-2 | SR-01000000089-4 |
| SR-01000000089-7 | SW197633-2 | TC-154347 |
| Taloxa | Tocris-0869 | Tox21_110145 |
| Tox21_110145_1 | Tox21_302368 | Tox21_500524 |
| UNII-X72RBB02N8 | W 554 | W-554 |
| WKGXYQFOCVYPAC-UHFFFAOYSA-N | X72RBB02N8 | Z1541638522 |
| ZINC1530803 | carbamic acid (3-carbamoyloxy-2-phenylpropyl) ester | carbamic acid 2-phenyltrimethylene ester |
| felbamate | s1330 |