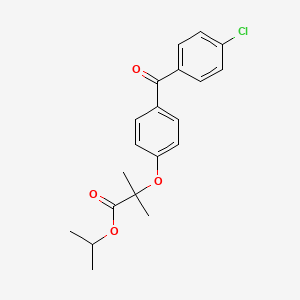
D0055 | Fenofibrate
C
C10BA04 Simvastatin and fenofibrate
[C10BA] HMG CoA reductase inhibitors in combination with other lipid modifying agents
[C10B] LIPID MODIFYING AGENTS, COMBINATIONS
[C10] LIPID MODIFYING AGENTS
[C] Cardiovascular system
C10BA03 Pravastatin and fenofibrate
[C10BA] HMG CoA reductase inhibitors in combination with other lipid modifying agents
[C10B] LIPID MODIFYING AGENTS, COMBINATIONS
[C10] LIPID MODIFYING AGENTS
[C] Cardiovascular system
C10AB05 Fenofibrate
[C10AB] Fibrates
[C10A] LIPID MODIFYING AGENTS, PLAIN
[C10] LIPID MODIFYING AGENTS
[C] Cardiovascular system
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Warning |
Aggregated GHS information provided by 281 companies from 13 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. Reported as not meeting GHS hazard criteria by 2 of 281 companies. For more detailed information, please visit ECHA C&L website Of the 12 notification(s) provided by 279 of 281 companies with hazard statement code(s): H373 (92.47%): Causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure] H413 (91.76%): May cause long lasting harmful effects to aquatic life [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P260, P273, P314, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| 1-methylethyl 2-[4-(4-chlorobenzoyl)phenoxy]-2-methylpropanoate | 2-(4-(4-Chlorobenzoyl)phenoxy)-2-methylpropanoic acid 1-methylethyl ester | 2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropanoic acid 1-methyl-ethyl ester |
| 2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropanoic acid 1-methylethyl ester | 2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropanoic acid isopropyl ester | 2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropionic Acid Isopropyl Ester |
| 2-[4-(4-chlorobenzoyl)phenoxy]-2-methyl-propanoic acid,1-methylethylester | 2-[4-(4-chlorobenzoyl)phenoxy]-2-methylpropanoicacidisopropylester | 2-[4-[(4-chlorophenyl)-oxomethyl]phenoxy]-2-methylpropanoic acid propan-2-yl ester |
| 28073-EP2272825A2 | 28073-EP2272841A1 | 28073-EP2277565A2 |
| 28073-EP2277566A2 | 28073-EP2277567A1 | 28073-EP2277568A2 |
| 28073-EP2277569A2 | 28073-EP2277570A2 | 28073-EP2287163A1 |
| 28073-EP2287165A2 | 28073-EP2287166A2 | 28073-EP2292280A1 |
| 28073-EP2292620A2 | 28073-EP2295409A1 | 28073-EP2298742A1 |
| 28073-EP2298776A1 | 28073-EP2301923A1 | 28073-EP2301936A1 |
| 28073-EP2305678A1 | 28073-EP2314588A1 | 49562-28-9 |
| 562F289 | A827746 | AB00052196 |
| AB00052196-15 | AB00052196-16 | AB00052196_17 |
| AB00052196_18 | AB0012237 | AB03716 |
| AB2000298 | AC-4227 | AK-88651 |
| AKOS005107777 | ALBB-028958 | ANW-41658 |
| API0003184 | API0025994 | API0026002 |
| API0026009 | AX8021009 | Ankebin |
| Antara | Antara (TN) | Antara (micronized) |
| BCP21243 | BDBM50085042 | BG0483 |
| BIDD:GT0574 | BPBio1_000166 | BRD-K50388907-001-05-6 |
| BRD-K50388907-001-18-9 | BRD-K50388907-001-20-5 | BRN 2062462 |
| BSPBio_000150 | BSPBio_003162 | C-20905 |
| C07586 | C20H21ClO4 | CAS-49562-28-9 |
| CC-28385 | CCG-38996 | CCRIS 7282 |
| CHEBI:5001 | CHEMBL672 | CIP-Fenofibrate |
| CPD000058299 | CS-0892 | CTK8B3039 |
| Certified Reference Material | D00565 | DB-051642 |
| DB01039 | DSSTox_CID_9874 | DSSTox_GSID_29874 |
| DSSTox_RID_78828 | DTXSID2029874 | DivK1c_000557 |
| EBD45918 | EC 256-376-3 | EINECS 256-376-3 |
| EU-0100486 | Elasterate | Elasterin |
| F 6020 | F0674 | FENOFIBRATE (MICRONIZED) |
| FENOFIBRATE (MICRONIZED) (fenofibrate | FNF | FT-0626400 |
| FT-0654669 | Fenobrate | Fenofibrate (JAN/INN) |
| Fenofibrate (Tricor, Trilipix) | Fenofibrate IDD-P | Fenofibrate [INN:BAN] |
| Fenofibrate [USAN:USP:INN:BAN] | Fenofibrate(Tricor) | Fenofibrate, 98% |
| Fenofibrate, >=99%, powder | Fenofibrate, European Pharmacopoeia (EP) Reference Standard | Fenofibrate, Pharmaceutical Secondary Standard |
| Fenofibrate, United States Pharmacopeia (USP) Reference Standard | Fenofibrate, analytical reference material | Fenofibrate,(S) |
| Fenofibrato | Fenofibrato [INN-Spanish] | Fenofibratum |
| Fenofibratum [INN-Latin] | Fenogal | Fenoglide |
| Fenomax | Fenotard | Finofibrate |
| Fulcro | GP4850 | GRS-027 |
| GTPL7186 | HMS1568H12 | HMS1921B17 |
| HMS2090G20 | HMS2092B05 | HMS2095H12 |
| HMS2231B14 | HMS3259K03 | HMS3261B13 |
| HMS3369M13 | HMS3649D20 | HMS3655K12 |
| HMS3712H12 | HMS501L19 | HSDB 7736 |
| HY-17356 | IDI1_000557 | Isopropyl (4''-(p-chlorobenzoyl)-2-phenoxy-2-methyl)propionate |
| Isopropyl (4'-(p-chlorobenzoyl)-2-phenoxy-2-methyl)propionate | Isopropyl 2-(4-(4-chlorobenzoyl)phenoxy)-2-methylpropanoate | Isopropyl 2-(4-(4-chlorobenzoyl)phenoxy)-2-methylpropionate |
| Isopropyl 2-(p-(p-chlorobenzoyl)phenoxy)-2-methylpropionate | Isopropyl 2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropionate | Isopropyl 2-[p-(p-chlorobenzoyl)phenoxy]-2-methylpropionate |
| J10318 | K-9221 | KBio1_000557 |
| KBio2_001730 | KBio2_004298 | KBio2_006866 |
| KBio3_002382 | KBioGR_000706 | KBioSS_001730 |
| KS-00000655 | LCP-Feno | LCP-FenoChol |
| LF 178 | LF-178 | LP00486 |
| LS-121256 | Lipanthyl | Lipantil |
| Lipantil (TN) | Lipidex | Lipidil |
| Lipifen | Lipirex | Lipoclar |
| Lipofen | Lipofen (TN) | Lipofene |
| Liposit | Lipsin | Lofibra |
| Lopac-F-6020 | Lopac0_000486 | Luxacor |
| MCULE-9460650238 | MFCD00133314 | MLS000028515 |
| MLS001148191 | MLS002548878 | MS-2223 |
| NC00452 | NCGC00015437-01 | NCGC00015437-02 |
| NCGC00015437-03 | NCGC00015437-04 | NCGC00015437-05 |
| NCGC00015437-06 | NCGC00015437-07 | NCGC00015437-08 |
| NCGC00015437-09 | NCGC00015437-10 | NCGC00015437-11 |
| NCGC00015437-12 | NCGC00015437-13 | NCGC00015437-14 |
| NCGC00015437-16 | NCGC00015437-17 | NCGC00021475-03 |
| NCGC00021475-04 | NCGC00021475-05 | NCGC00021475-06 |
| NCGC00021475-07 | NCGC00021475-08 | NCGC00253945-01 |
| NCGC00261171-01 | NINDS_000557 | NSC 281319 |
| NSC-281319 | NSC-757822 | NSC281319 |
| NSC757822 | Nolipax | Opera_ID_328 |
| Pharmakon1600-01501010 | Pharmavit | Phenofibrate |
| Prestwick0_000275 | Prestwick1_000275 | Prestwick2_000275 |
| Prestwick3_000275 | Prestwick_217 | Procetofen |
| Procetofene | Procetoken | Proctofene |
| Propanoic acid, 2-(4-(4-chlorobenzoyl)phenoxy)-2-methyl-, 1-methylethyl ester | Propanoic acid, 2-[4-(4-chlorobenzoyl)phenoxy]-2-methyl-, 1-methylethyl ester | Propanoic acid, 2-[4-(4-chlorobenzoyl)phenoxy]-2-methyl-, 1-methylethylester |
| Protolipan | PubChem17489 | Q-201111 |
| Q419724 | SAM002264613 | SBB037926 |
| SBI-0050470.P003 | SCHEMBL4670 | SMR000058299 |
| SPBio_001380 | SPBio_002369 | SPECTRUM1501010 |
| SR-01000000091 | SR-01000000091-16 | SR-01000000091-2 |
| SR-01000000091-5 | SR-01000000091-6 | SR-01000000091-8 |
| ST085313 | SW196525-4 | Secalip |
| Sedufen | Spectrum2_001390 | Spectrum3_001431 |
| Spectrum4_000413 | Spectrum5_001479 | Spectrum_001250 |
| Supralip | TC-130403 | TRICOR (MICRONIZED) |
| Tox21_110147 | Tox21_110147_1 | Tox21_300151 |
| Tox21_500486 | Tricor | Tricor (TN) |
| Triglide | Triglide (TN) | U202363UOS |
| UNII-U202363UOS | YMTINGFKWWXKFG-UHFFFAOYSA-N | Z2768724415 |
| ZINC584092 | ZX-AN079771 | fenofibrate |
| fenofibrate; | isopropyl 2-[4-(4-chlorobenzoyl)phenoxy]-2-methyl-propanoate | isopropyl 2-[4-(4-chlorobenzoyl)phenoxy]-2-methylpropanoate |
| methylethyl 2-{4-[(4-chlorophenyl)carbonyl]phenoxy}-2-methylpropanoate | propan-2-yl 2-[4-(4-chlorobenzoyl)phenoxy]-2-methylpropanoate | propan-2-yl 2-[4-(4-chlorophenyl)carbonylphenoxy]-2-methyl-propanoate |
| propan-2-yl 2-{4-[(4-chlorophenyl)carbonyl]phenoxy}-2-methylpropanoate | s1794 |
| DrugBank Name | Fenofibrate |
| DrugBank | DB01039 |
| CAS Number | 1092484-56-4, 49562-28-9, 675576-98-4 |
| PubChem Compound | 3339 |
| KEGG Compound ID | C07586 |
| KEGG Drug | D00565 |
| PubChem.Substance | 46507371 |
| ChEBI | 5001 |
| PharmGKB | PA449594 |
| ChemSpider | 3222 |
| BindingDB | 50085042.0 |
| TTD | DAP000270 |
| Wikipedia | Fenofibrate |
| DPD | 1381 |