D0056 | Fenoprofen
M
M01AE04 Fenoprofen
[M01AE] Propionic acid derivatives
[M01A] ANTIINFLAMMATORY AND ANTIRHEUMATIC PRODUCTS, NON-STEROIDS
[M01] ANTIINFLAMMATORY AND ANTIRHEUMATIC PRODUCTS
[M] Musculoskeletal system
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Warning |
Aggregated GHS information provided by 40 companies from 2 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] H312 (97.5%): Harmful in contact with skin [Warning Acute toxicity, dermal] H332 (97.5%): Harmful if inhaled [Warning Acute toxicity, inhalation] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P312, P322, P330, P363, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
 |
Warning |
The GHS information provided by 1 company from 1 notification to the ECHA C&L Inventory. H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] |
P264, P270, P301+P312, P330, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD | oral | > 200mg/kg (200mg/kg) | gastrointestinal: ulceration or bleeding from stomach | Toxicology and Applied Pharmacology. Vol. 52, Pg. 454, 1980. |
| rat | LD50 | oral | 415mg/kg (415mg/kg) | Kiso to Rinsho. Clinical Report. Vol. 14, Pg. 4385, 1980. | |
| human | TDLo | oral | 4286ug/kg (4.286mg/kg) | endocrine: evidence of thyroid hyperfunction | British Medical Journal. Vol. 292, Pg. 1560, 1986. |
| women | TDLo | oral | 720mg/kg/30D- (720mg/kg) | kidney, ureter, and bladder: "changes in tubules (including acute renal failure, acute tubular necrosis)" | JAMA, Journal of the American Medical Association. Vol. 242, Pg. 1896, 1979. |
| man | TDLo | oral | 521mg/kg/17W- (521mg/kg) | Annals of Internal Medicine. Vol. 93, Pg. 508, 1980. | |
| women | TDLo | oral | 1898mg/kg/12W (1898mg/kg) | Annals of Internal Medicine. Vol. 92, Pg. 72, 1980. | |
| rat | LD50 | intravenous | 526mg/kg (526mg/kg) | Yakkyoku. Pharmacy. Vol. 34, Pg. 363, 1983. | |
| rat | LD50 | intraperitoneal | 234mg/kg (234mg/kg) | Yakkyoku. Pharmacy. Vol. 34, Pg. 363, 1983. | |
| mouse | LD50 | oral | 439mg/kg (439mg/kg) | Yaoxue Tongbao. Bulletin of Pharmacology. Vol. 21, Pg. 36, 1986. | |
| mouse | LD50 | oral | 1400mg/kg (1400mg/kg) | United States Patent Document. Vol. #3985779, | |
| mouse | LD50 | subcutaneous | 463mg/kg (463mg/kg) | Yakkyoku. Pharmacy. Vol. 34, Pg. 363, 1983. | |
| rat | LD50 | subcutaneous | 366mg/kg (366mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 13, Pg. 1128, 1982. | |
| women | TDLo | oral | 9386mg/kg/1Y- (9386mg/kg) | kidney, ureter, and bladder: interstitial nephritis | New England Journal of Medicine. Vol. 301, Pg. 1271, 1979. |
| mouse | LD50 | intravenous | 471mg/kg (471mg/kg) | Yakkyoku. Pharmacy. Vol. 34, Pg. 363, 1983. | |
| women | LDLo | oral | 8748mg/kg/35W (8748mg/kg) | American Journal of Medicine. Vol. 72, Pg. 81, 1982. | |
| women | TDLo | oral | 5832mg/kg/35W (5832mg/kg) | American Journal of Medicine. Vol. 72, Pg. 81, 1982. | |
| women | TDLo | oral | 1092mg/kg/13W (1092mg/kg) | Mayo Clinic Proceedings. Vol. 55, Pg. 103, 1980. | |
| mouse | LD50 | intraperitoneal | 286mg/kg (286mg/kg) | Yakkyoku. Pharmacy. Vol. 34, Pg. 363, 1983. | |
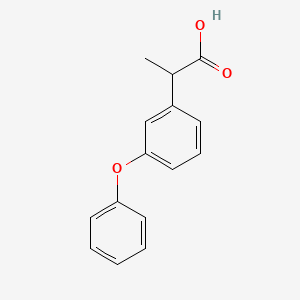
| (+-)-2-(3-Phenoxyphenyl)propionic acid | (+-)-m-Phenoxyhydratropic acid | (+/-)-Fenoprofen |
| (+/-)-m-phenoxyhydratropic acid | (.+/-.)-m-Phenoxyhydratropic acid | 2-(3-Phenoxy-phenyl)-propionic acid |
| 2-(3-Phenoxyphenyl)propanoic acid | 2-(3-Phenoxyphenyl)propanoic acid # | 2-(3-Phenoxyphenyl)propionic acid |
| 2-(3-phenoxyphenyl)-propionic acid | 2-(m-phenoxyphenyl)propionic acid | 2-[3-(phenyloxy)phenyl]propanoic acid |
| 29679-58-1 | 3-Phenoxyhydratropic acid | 3-phenoxy-hydratropic acid |
| 31879-05-7 | 4CH-007316 | 53746-45-5 |
| 879F057 | AB00052197 | AB00052197-04 |
| AB00052197_05 | AB00052197_06 | AB0065019 |
| AK116448 | AKOS005256238 | AS-30559 |
| BBL100921 | BDBM54705 | BPBio1_000846 |
| BRN 2118687 | BSPBio_000768 | Benzeneacetic acid, .alpha.-methyl-3-phenoxy-, (.+/-.)- |
| Benzeneacetic acid, a-methyl-3-phenoxy-, (as)- | Benzeneacetic acid, alpha-methyl-3-phenoxy- | Benzeneacetic acid, alpha-methyl-3-phenoxy-, (+-)- |
| Benzeneacetic acid, |A-methyl-3-phenoxy- | C06997 | CCG-39009 |
| CHEBI:5004 | CHEMBL1297 | CTK4G3649 |
| D02350 | DB00573 | DL-2-(3-phenoxyphenyl)propionic acid |
| DS-017185 | DTXSID9023045 | Di-benzeneacetate, .alpha.-methyl-3-phenoxy-, calcium salt, dihydrate |
| DivK1c_000848 | EINECS 249-770-1 | EINECS 250-850-3 |
| Epitope ID:139975 | Feneprofen calcium salt dihydrate | Fenoprofen (USAN/INN) |
| Fenoprofen Dihydrate, Calcium Salt | Fenoprofen [USAN:BAN:INN] | Fenoprofen [USAN:INN:BAN] |
| Fenoprofen calcium | Fenoprofen calcium hydrate | FenoprofenCa |
| Fenoprofene | Fenoprofene [INN-French] | Fenoprofeno |
| Fenoprofeno [INN-Spanish] | Fenoprofenum | Fenoprofenum [INN-Latin] |
| GTPL4820 | HMS1921B19 | HMS2090G18 |
| HMS2092B07 | HMS502K10 | HSDB 3328 |
| Hydratropic acid, m-phenoxy-, (+-)- | Hydratropic acid, m-phenoxy-, (.+/-.)- | IDI1_000848 |
| J-018559 | KBio1_000848 | KBio2_001732 |
| KBio2_004300 | KBio2_006868 | KBio3_001860 |
| KBioGR_001477 | KBioSS_001732 | LS-76362 |
| Lilly 53838 | Lilly 53858 | Lilly-53858 |
| M-8808 | MCULE-2686503876 | N6-[2-[[4-(Diethylamino)-1-methylbu-tyl]amino]-6-methyl-4-pyrimidinyl]-2-methyl-4,6-qu-inolinediamine trihydrochloride |
| NCGC00094887-01 | NCGC00094887-02 | NCGC00094887-03 |
| NINDS_000848 | NSC-757813 | NSC757813 |
| Nalfon | Pharmakon1600-01501011 | Prestwick0_000754 |
| Prestwick1_000754 | Prestwick2_000754 | Prestwick3_000754 |
| Q2555245 | RDJGLLICXDHJDY-UHFFFAOYSA-N | SBI-0051632.P002 |
| SCHEMBL3797 | SPBio_001402 | SPBio_002707 |
| SPECTRUM1501011 | SR-05000001767 | SR-05000001767-1 |
| ST24027516 | STL554715 | Spectrum2_001391 |
| Spectrum3_000910 | Spectrum4_001009 | Spectrum5_001311 |
| Spectrum_001252 | UNM-0000306101 | Z4588 |
| alpha-(m-phenoxyphenyl)-propionic acid | alpha-(m-phenoxyphenyl)propionic acid | alpha-Methyl-3-phenoxybenzeneacetic acid |
| calcium;2-(3-phenoxyphenyl)propanoic acid;hydrate | calcium;2-(3-phenoxyphenyl)propionic acid;hydrate | cid_16219353 |
| fenoprofen |
| DrugBank Name | Fenoprofen |
| DrugBank | DB00573 |
| CAS Number | 1177865-17-6, 29679-58-1, 31879-05-7, 34597-40-5, 53746-45-5, 71720-56-4, 95907-05-4 |
| PubChem Compound | 3342 |
| KEGG Compound ID | C06997 |
| KEGG Drug | D00968 |
| PubChem.Substance | 46504597 |
| ChEBI | 5004 |
| PharmGKB | PA449597 |
| ChemSpider | 3225 |
| BindingDB | 54705.0 |
| TTD | DAP000619 |
| Wikipedia | Fenoprofen |
| DPD | 11356 |