Drug
D0068 | Imipenem
J
J01DH51 Imipenem and cilastatin
[J01DH] Carbapenems
[J01D] OTHER BETA-LACTAM ANTIBACTERIALS
[J01] ANTIBACTERIALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| RESPIRATION | inhibit | 197 | ||||||
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | intravenous | > 1316mg/kg (1316mg/kg) | Drugs in Japan Vol. -, Pg. 134, 1990. | |
| rat | LD50 | subcutaneous | 2gm/kg (2000mg/kg) | Drugs in Japan Vol. -, Pg. 134, 1990. | |
| rat | LD50 | oral | > 5gm/kg (5000mg/kg) | Drugs in Japan Vol. -, Pg. 134, 1990. | |
| mouse | LD50 | intravenous | 664ug/kg (0.664mg/kg) | Journal of Antibiotics. Vol. 48, Pg. 408, 1995. | |
| mouse | LD50 | intravenous | 1068mg/kg (1068mg/kg) | Drugs in Japan Vol. -, Pg. 134, 1990. | |
| mouse | LD50 | oral | > 5gm/kg (5000mg/kg) | Drugs in Japan Vol. -, Pg. 134, 1990. | |
| rat | LD50 | oral | > 5gm/kg (5000mg/kg) | gastrointestinal: "hypermotility, diarrhea" | Chemotherapy Vol. 33(Suppl, |
| rat | LD50 | subcutaneous | > 5gm/kg (5000mg/kg) | Chemotherapy Vol. 33(Suppl, | |
| mouse | LD50 | subcutaneous | > 5gm/kg (5000mg/kg) | Chemotherapy Vol. 33(Suppl, | |
| mouse | LD50 | subcutaneous | 1922mg/kg (1922mg/kg) | Drugs in Japan Vol. -, Pg. 134, 1990. | |
| rat | LD50 | intravenous | 1972mg/kg (1972mg/kg) | Chemotherapy Vol. 33(Suppl, | |
| mouse | LD50 | oral | > 5gm/kg (5000mg/kg) | gastrointestinal: "hypermotility, diarrhea" | Chemotherapy Vol. 33(Suppl, |
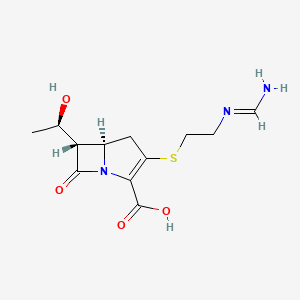
| (5R,6S)-3-((2-(Formimidoylamino)ethyl)thio)-6-((R)-1-hydroxyethyl)-7-oxo-1-azabicyclo(3.2.0)hept-2-ene-2-carboxylic acid | (5R,6S)-3-((2-formimidamidoethyl)thio)-6-((R)-1-hydroxyethyl)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid | (5R,6S)-3-(2-Formimidoylamino-ethylsulfanyl)-6-((R)-1-hydroxy-ethyl)-7-oxo-1-aza-bicyclo[3.2.0]hept-2-ene-2-carboxylic acid |
| (5R,6S)-3-(2-formimidamidoethylthio)-6-((R)-1-hydroxyethyl)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid | (5R,6S)-3-({2-[(E)-(aminomethylidene)amino]ethyl}sulfanyl)-6-[(1R)-1-hydroxyethyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid | (5R,6S)-6-((R)-1-Hydroxyethyl)-3-(2-(iminomethylamino)ethylthio)-7-oxo-1-azabicyclo(3.2.0)hept-2-ene-2-carbonsaeure |
| (5R,6S)-6-[(1R)-1-hydroxyethyl]-3-(2-methanimidamidoethylsulfanyl)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid | (5R,6S)-6-[(1R)-1-hydroxyethyl]-3-({2-[(iminomethyl)amino]ethyl}sulfanyl)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid | (5R,6S)-6-[(1R)-1-hydroxyethyl]-3-({2-[(iminomethyl)amino]ethyl}thio)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid |
| (5R,6S)-6-[(1R)-1-hydroxyethyl]-3-[(2-methanimidamidoethyl)sulfanyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid | 1-Azabicyclo(3.2.0)hept-2-ene-2-carboxylic acid, 6-((1R)-1-hydroxyethyl)-3-((2-((iminomethyl)amino)ethyl)thio)-7-oxo-, (5R,6S)- | 1-Azabicyclo(3.2.0)hept-2-ene-2-carboxylic acid, 6-(1-hydroxyethyl)-3-((2-((iminomethyl)amino)ethyl)thio)-7-oxo-, (5R-(5-alpha,6-alpha(R*)))- |
| 1-Azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid, 6-[(1R)-1-hydroxyethyl]-3-[[2-[(iminomethyl)amino]ethyl]thio]-7-oxo-, (5R,6S)- | 1-Azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid,6-[(1R)-1-hydroxyethyl]-3-[[2-[(iminomethyl)amino]ethyl]thio]-7-oxo-,(5R,6S)- | 103730-39-8 |
| 3-[(2-Aminoethyl)thio]-6-[(1R)-1-hydroxyethyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid (4-nitrophenyl)methylester monohydrochloride compd. with 1-methyl-2-pyrrolidinone (1:1) | 64221-86-9 | 74431-23-5 |
| 847I667 | AB01563339_01 | AKOS016010844 |
| AS-75130 | BCP13012 | BDBM50049708 |
| BDBM50213266 | BIDD:GT0686 | BPBio1_000525 |
| BSPBio_000477 | C06665 | CAS-64221-86-9 |
| CCG-220519 | CCG-221583 | CHEBI:471744 |
| CHEMBL148 | D04515 | DB01598 |
| DSSTox_CID_3143 | DSSTox_GSID_23143 | DSSTox_RID_76888 |
| DTXSID2023143 | EINECS 264-734-5 | Epitope ID:120384 |
| FT-0627191 | HMS1569H19 | HMS2090A15 |
| HMS2096H19 | HMS3260H20 | HMS3713H19 |
| Imipemide | Imipenem (INN) | Imipenem [INN] |
| Imipenem anhydrous | Imipenem, N-Formimidoyl thienamycin | Imipenemum |
| Imipenemum [Latin] | Imipenen | LP00279 |
| MK 0787 | MK-0787 | MK-787 |
| N-Formimidoylthienamycin | N-formimidoyl thienamycin | NCGC00016928-01 |
| NCGC00167958-01 | NCGC00167958-02 | NCGC00167958-03 |
| NCGC00167958-05 | NCGC00260964-01 | NSC-717864 |
| NSC-759901 | NSC717864 | NSC759901 |
| Pharmakon1600-01506001 | Prestwick0_000519 | Prestwick1_000519 |
| Prestwick2_000519 | Prestwick3_000519 | Prestwick_844 |
| Primaxin | Q20IM7HE75 | Q425152 |
| SCHEMBL1649260 | SCHEMBL8781920 | SPBio_002398 |
| SR-05000000294 | SR-05000000294-2 | SR-05000000294-5 |
| Thienamycin p-nitrobenzylester hydrochloride (N-methylpyrrolidinonesolvate) | Tienamycin | Tox21_110689 |
| Tox21_110689_1 | Tox21_500279 | UNII-Q20IM7HE75 |
| X-2302 | ZINC4097225 | ZSKVGTPCRGIANV-ZXFLCMHBSA-N |
| [5R-[5.alpha.,6.alpha.(R*)]]-6-(1-Hydroxyethyl)-3-[[2- [(iminomethyl)amino]ethyl]thio]-7-oxo-1-azabicyclo[3.2.0]hept-2- ene-2-carboxylic acid monohydrate | imipen | imipenem |
| DrugBank Name | Imipenem |
| DrugBank | DB01598 |
| CAS Number | 103730-39-8, 442847-66-7, 64221-86-9, 6623-14-9, 74431-23-5, 85960-17-4 |
| PubChem Compound | 104838 |
| KEGG Compound ID | C06665 |
| KEGG Drug | D04515 |
| PubChem.Substance | 46505744 |
| ChEBI | 471744 |
| PharmGKB | PA449968 |
| ChemSpider | 94631 |
| BindingDB | 50049708.0 |
| TTD | DAP000459 |
| Wikipedia | Imipenem |
| DPD | 1390 |
1. Vuda et al. (2016)