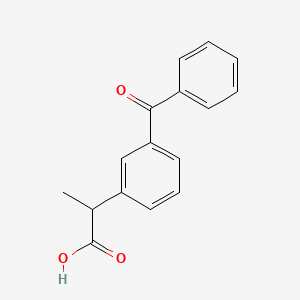
D0074 | Ketoprofen
M
M02AA10 Ketoprofen
[M02AA] Antiinflammatory preparations, non-steroids for topical use
[M02A] TOPICAL PRODUCTS FOR JOINT AND MUSCULAR PAIN
[M02] TOPICAL PRODUCTS FOR JOINT AND MUSCULAR PAIN
[M] Musculoskeletal system
M01AE53 Ketoprofen, combinations
[M01AE] Propionic acid derivatives
[M01A] ANTIINFLAMMATORY AND ANTIRHEUMATIC PRODUCTS, NON-STEROIDS
[M01] ANTIINFLAMMATORY AND ANTIRHEUMATIC PRODUCTS
[M] Musculoskeletal system
M01AE03 Ketoprofen
[M01AE] Propionic acid derivatives
[M01A] ANTIINFLAMMATORY AND ANTIRHEUMATIC PRODUCTS, NON-STEROIDS
[M01] ANTIINFLAMMATORY AND ANTIRHEUMATIC PRODUCTS
[M] Musculoskeletal system
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Danger |
Aggregated GHS information provided by 637 companies from 20 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. Reported as not meeting GHS hazard criteria by 1 of 637 companies. For more detailed information, please visit ECHA C&L website Of the 19 notification(s) provided by 636 of 637 companies with hazard statement code(s): H301 (99.84%): Toxic if swallowed [Danger Acute toxicity, oral] H315 (23.11%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (23.11%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (23.27%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P261, P264, P270, P271, P280, P301+P310, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| human | TDLo | oral | 714ug/kg (0.714mg/kg) | gastrointestinal: nausea or vomiting | Journal of Clinical Pharmacology. Vol. 24, Pg. 486, 1984. |
| women | TDLo | oral | 11mg/kg/12H-I (11mg/kg) | Journal of Clinical Pyschopharmacology. Vol. 19, Pg. 95, 1999. | |
| guinea pig | LD50 | oral | 1300mg/kg (1300mg/kg) | Journal de Pharmacologie. Vol. 2, Pg. 259, 1971. | |
| guinea pig | LD50 | intravenous | 450mg/kg (450mg/kg) | Journal de Pharmacologie. Vol. 2, Pg. 259, 1971. | |
| mouse | LD50 | oral | 360mg/kg (360mg/kg) | Polish Journal of Pharmacology and Pharmacy. Vol. 38, Pg. 107, 1986. | |
| mouse | LD50 | subcutaneous | 550mg/kg (550mg/kg) | Journal de Pharmacologie. Vol. 2, Pg. 259, 1971. | |
| rat | LD50 | intravenous | 350mg/kg (350mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 9, Pg. 222, 1978. | |
| mouse | LD50 | intravenous | 500mg/kg (500mg/kg) | Journal de Pharmacologie. Vol. 2, Pg. 259, 1971. | |
| child | TDLo | unreported | 300mg/kg/15D- (300mg/kg) | New England Journal of Medicine. Vol. 300, Pg. 796, 1979. | |
| rat | LD50 | oral | 62400ug/kg (62.4mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 34, Pg. 280, 1984. | |
| women | TDLo | oral | 80mg/kg/10D-I (80mg/kg) | kidney, ureter, and bladder: "changes in tubules (including acute renal failure, acute tubular necrosis)" | British Medical Journal. Vol. 292, Pg. 97, 1986. |
| mouse | LD50 | intraperitoneal | 300mg/kg (300mg/kg) | European Journal of Medicinal Chemistry--Chimie Therapeutique. Vol. 11, Pg. 7, 1976. | |
| man | TDLo | oral | 5714ug/kg (5.714mg/kg) | Clinical Rheumatology. Vol. 10, Pg. 215, 1991. | |
| man | TDLo | oral | 5714ug/kg (5.714mg/kg) | liver: "hepatitis (hepatocellular necrosis), zonal" | Clinical Rheumatology. Vol. 10, Pg. 215, 1991. |
| rat | LD50 | subcutaneous | 100mg/kg (100mg/kg) | Journal de Pharmacologie. Vol. 2, Pg. 259, 1971. | |
| rat | LD50 | intraperitoneal | 80mg/kg (80mg/kg) | Drugs in Japan Vol. 6, Pg. 265, 1982. | |
| rat | LD50 | rectal | 84mg/kg (84mg/kg) | Journal of Toxicological Sciences. Vol. 6, Pg. 209, 1981. | |
| (+) ketoprofen | (+-)-3-Benzoyl-alpha-methylbenzeneacetic acid | (+-)-m-Benzoylhydratropic acid |
| (+/-)-2-(3-Benzoylphenyl)propionic acid | (+/-)-m-Benzoylhydratropic acid | (.+/-.)-3-Benzoyl-.alpha.-methylbenzeneacetic acid |
| (.+/-.)-m-Benzoylhydratropic acid | (2R)-2-(3-benzoylphenyl)propanoic acid | (rs)-2-(3-benzoylphenyl)propanoic acid |
| 071K154 | 19,583 RP | 19583 RP |
| 2-(3'-benzoylphenyl)propionic acid | 2-(3-Benzoylphenyl)propanoic acid | 2-(3-Benzoylphenyl)propanoic acid # |
| 2-(3-Benzoylphenyl)propionic acid | 2-(3-benzoylphenyl) propionic acid | 2-(3-benzoylphenyl) propionoic acid |
| 2-(3-benzoylphenyl)-propionic acid | 2-(m-Benzoylphenyl)propionic acid | 2-[3-(benzoyl)phenyl]propanoic acid |
| 2-[3-(phenylcarbonyl)phenyl]propanoic acid | 22071-15-4 | 22161-86-0 |
| 3-BENZOYL-ALPHA-METHYLBENZENEACETIC ACID | 3-Benzoyl-.alpha.-methylbenzeneacetic acid | 3-Benzoylhydratropic acid |
| A815896 | AB00052249 | AB00052249-17 |
| AB00052249-19 | AB00052249-20 | AB00052249_21 |
| AB00052249_22 | AB2000190 | AC-1486 |
| AK-47496 | AKOS007930512 | AM20060549 |
| Acide (benzoyl-3-phenyl)-2-propionique | Acide (benzoyl-3-phenyl)-2-propionique [French] | Actron |
| Alrheumat | Alrheumum | Alrheumun |
| Aneol | BCP0726000302 | BCP23428 |
| BCP9000810 | BDBM50022271 | BIDD:GT0443 |
| BIM-0050664.0001 | BPBio1_000261 | BR-47496 |
| BRD-A97739905-001-05-9 | BRD-A97739905-001-15-8 | BSPBio_000237 |
| BSPBio_003037 | Benzeneacetic acid, 3-benzoyl-.alpha.-methyl- | Benzeneacetic acid, 3-benzoyl-?-methyl- |
| Benzeneacetic acid, 3-benzoyl-alpha-methyl- | Benzeneacetic acid, 3-benzoyl-alpha-methyl-, (+-)- | Benzeneacetic acid, 3-benzoyl-alpha-methyl-, (+/-) |
| Benzoylhydratropic Acid | C01716 | CAS-22071-15-4 |
| CCG-39685 | CCRIS 4508 | CHEBI:6128 |
| CHEMBL571 | CPD000040181 | CS-2175 |
| CTK8F2159 | Capisten | Certified Reference Material |
| D00132 | DB01009 | DKYWVDODHFEZIM-UHFFFAOYSA- |
| DKYWVDODHFEZIM-UHFFFAOYSA-N | DSSTox_CID_771 | DSSTox_GSID_20771 |
| DSSTox_RID_75783 | DTXSID6020771 | Dexal |
| DivK1c_000598 | EINECS 244-759-8 | EU-0100686 |
| Epatec | Epitope ID:131783 | F2173-0960 |
| FT-0082352 | FT-0602834 | Fastum |
| GTPL4795 | HMS1568L19 | HMS1921B12 |
| HMS2089B16 | HMS2092L19 | HMS2095L19 |
| HMS2234H16 | HMS3259I05 | HMS3262I13 |
| HMS3372M08 | HMS3373G09 | HMS3649N10 |
| HMS3655C15 | HMS3712L19 | HMS501N20 |
| HY-B0227 | Hydratropic acid, m-benzoyl-, (+-)- | IDI1_000598 |
| InChI=1/C16H14O3/c1-11(16(18)19)13-8-5-9-14(10-13)15(17)12-6-3-2-4-7-12/h2-11H,1H3,(H,18,19) | Iso-K | K 1751 |
| K0038 | KBio1_000598 | KBio2_001789 |
| KBio2_004357 | KBio2_006925 | KBio3_002537 |
| KBioGR_000435 | KBioSS_001789 | KS-00000GGJ |
| KS-5031 | Kefenid | Ketoprofen (+-) |
| Ketoprofen (Actron) | Ketoprofen (JP17/USP/INN) | Ketoprofen ,(S) |
| Ketoprofen [USAN:INN:BAN:JAN] | Ketoprofen [USAN:USP:INN:BAN:JAN] | Ketoprofen(Actron) |
| Ketoprofen, >=98% (TLC) | Ketoprofen, British Pharmacopoeia (BP) Reference Standard | Ketoprofen, European Pharmacopoeia (EP) Reference Standard |
| Ketoprofen, Pharmaceutical Secondary Standard | Ketoprofen, United States Pharmacopeia (USP) Reference Standard | Ketoprofen, VETRANAL(TM), analytical standard |
| Ketoprofen, meets USP testing specifications | Ketoprofene | Ketoprofene [INN-French] |
| Ketoprofeno | Ketoprofeno [INN-Spanish] | Ketoprofenum |
| Ketoprofenum [INN-Latin] | Ketopron | Ketoprophene |
| L''Acide (benzoyl-3-phenyl)-2-propionique | L'Acide (benzoyl-3-phenyl)-2-propionique | LP00686 |
| LS-28621 | LS-7470 | Lertus |
| Lopac0_000686 | M-7468 | MCULE-9740144074 |
| MFCD00055790 | MLS000028446 | MLS000079024 |
| MLS001201752 | MLS001306444 | MLS002548889 |
| MLS006011967 | Menamin | Meprofen |
| NC00459 | NCGC00015578-02 | NCGC00015578-03 |
| NCGC00015578-04 | NCGC00015578-05 | NCGC00015578-07 |
| NCGC00015578-08 | NCGC00015578-10 | NCGC00015578-12 |
| NCGC00016757-01 | NCGC00094043-01 | NCGC00094043-02 |
| NCGC00094043-03 | NCGC00094043-04 | NCGC00258401-01 |
| NCGC00261371-01 | NINDS_000598 | NSC-758144 |
| NSC758144 | Opera_ID_509 | Oprea1_117113 |
| Orudis | Orudis (TN) | Orudis KT |
| Orudis, Profenid, Dexal, Keduril, Ketofen, | Orudis;Oruvail;Ketoflam;Orudis KT;Actron | Orugesic |
| Oruvail | Oscorel | Pharmakon1600-01501215 |
| Prestwick0_000219 | Prestwick1_000219 | Prestwick2_000219 |
| Prestwick3_000219 | Prestwick_617 | Profenid |
| Propionic acid, 2-(3-benzoylphenyl)- | Q-201268 | Q409192 |
| R.P. 19,583 | R.P. 19583 | RP 19583 |
| RP-19583 | RU 4733 | Racemic ketoprofen |
| SAM002264620 | SBB063946 | SBI-0050664.P003 |
| SC-17621 | SCHEMBL2896 | SMR000040181 |
| SPBio_000952 | SPBio_002158 | SPECTRUM1501215 |
| SR-01000075949 | SR-01000075949-1 | SR-01000075949-18 |
| SR-01000075949-6 | SR-01000075949-9 | ST2409293 |
| STL450995 | SW196784-3 | Spectrum2_000956 |
| Spectrum3_001479 | Spectrum4_000028 | Spectrum5_001254 |
| Spectrum_001309 | TRA-0188004 | Toprec |
| Toprek | Tox21_110594 | Tox21_110594_1 |
| Tox21_200847 | Tox21_500686 | UNM-0000306100 |
| Z1695709452 | alpha(3-benzoylphenyl)propionic acid | alpha-(3-benzoylphenyl)propionic acid |
| alpha-(m-benzoylphenyl) propionic acid | alpha-(m-benzoylphenyl)propionic acid | ketoprofen |
| m-Benzoylhydratropic acid | m-benzoyl-hydratropic acid | racemic-Ketoprofen |
| s1645 |
| DrugBank Name | Ketoprofen |
| DrugBank | DB01009 |
| CAS Number | 1219805-29-4, 154907-35-4, 156604-79-4, 15962-46-6, 22071-15-4, 22161-81-5, 22161-86-0, 56105-81-8, 57469-78-0, 57495-14-4 |
| PubChem Compound | 3825 |
| KEGG Compound ID | C01716 |
| KEGG Drug | D00132 |
| PubChem.Substance | 46505715 |
| ChEBI | 6128 |
| PharmGKB | PA450149 |
| ChemSpider | 3693 |
| BindingDB | 50022271.0 |
| TTD | DAP000623 |
| Wikipedia | Ketoprofen |
| DPD | 2327 |