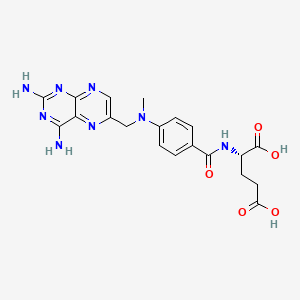
D0082 | Methotrexate
L
L04AX03 Methotrexate
[L04AX] Other immunosuppressants
[L04A] IMMUNOSUPPRESSANTS
[L04] IMMUNOSUPPRESSANTS
[L] Antineoplastic and immunomodulating agents
L01BA01 Methotrexate
[L01BA] Folic acid analogues
[L01B] ANTIMETABOLITES
[L01] ANTINEOPLASTIC AGENTS
[L] Antineoplastic and immunomodulating agents
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| MITOCHONDRIAL FATTY ACID BETA OXIDATION | 44μM | 42 | mice | Lean mice vs Ob/ob mice | Measurement of oxygen consumption in the presence of ADP (state 3) and the different substrates was carried out on the Mitologics screening platform | EC20 | 227 | |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
   |
Danger |
Aggregated GHS information provided by 252 companies from 12 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H301 (100%): Toxic if swallowed [Danger Acute toxicity, oral] H315 (98.41%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (98.81%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H340 (16.27%): May cause genetic defects [Danger Germ cell mutagenicity] H360 (98.41%): May damage fertility or the unborn child [Danger Reproductive toxicity] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P264, P270, P280, P281, P301+P310, P302+P352, P305+P351+P338, P308+P313, P321, P330, P332+P313, P337+P313, P362, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
   |
Danger |
Aggregated GHS information provided by 39 companies from 2 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H301 (100%): Toxic if swallowed [Danger Acute toxicity, oral] H315 (97.44%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (97.44%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H360 (100%): May damage fertility or the unborn child [Danger Reproductive toxicity] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P264, P270, P280, P281, P301+P310, P302+P352, P305+P351+P338, P308+P313, P321, P330, P332+P313, P337+P313, P362, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | unreported | 32mg/kg (32mg/kg) | Neoplasma. Vol. 29, Pg. 43, 1982. | |
| (+)-4-Amino-10-methylfolic acid | (2S)-2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)amino}phenyl)formamido]pentanedioic acid | (2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methyl-amino]benzoyl]amino]glutaric acid;hydrate |
| (2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methyl-amino]benzoyl]amino]pentanedioic acid | (2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methylamino]benzoyl]amino]pentanedioic acid | (2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methylamino]benzoyl]amino]pentanedioic acid;hydrate |
| (2S)-2-[[4-[[2,4-bis(azanyl)pteridin-6-yl]methyl-methyl-amino]phenyl]carbonylamino]pentanedioic acid;hydrate | (2S)-2-[[[4-[(2,4-diamino-6-pteridinyl)methyl-methylamino]phenyl]-oxomethyl]amino]pentanedioic acid;hydrate | (4-(((2,4-diaminopteridin-6-yl)methyl)(methyl)amino)benzoyl)-L-glutamic acid |
| (S)-2-(4-(((2,4-Diaminopteridin-6-yl)methyl)(methyl)amino)benzamido)pentanedioic acid | (S)-2-(4-(((2,4-diaminopteridin-6-yl)methyl) | (methyl)amino)benzamido)pentanedioic acid |
| 133073-73-1 | 1771-EP0930075A1 | 1771-EP2269989A1 |
| 1771-EP2269994A1 | 1771-EP2270001A1 | 1771-EP2270008A1 |
| 1771-EP2270018A1 | 1771-EP2270505A1 | 1771-EP2272825A2 |
| 1771-EP2272827A1 | 1771-EP2272832A1 | 1771-EP2275413A1 |
| 1771-EP2275420A1 | 1771-EP2277565A2 | 1771-EP2277566A2 |
| 1771-EP2277567A1 | 1771-EP2277568A2 | 1771-EP2277569A2 |
| 1771-EP2277570A2 | 1771-EP2277865A1 | 1771-EP2277876A1 |
| 1771-EP2280012A2 | 1771-EP2281563A1 | 1771-EP2281815A1 |
| 1771-EP2287156A1 | 1771-EP2289892A1 | 1771-EP2289894A2 |
| 1771-EP2292233A2 | 1771-EP2292280A1 | 1771-EP2292595A1 |
| 1771-EP2292614A1 | 1771-EP2292615A1 | 1771-EP2292617A1 |
| 1771-EP2295055A2 | 1771-EP2295416A2 | 1771-EP2295426A1 |
| 1771-EP2295427A1 | 1771-EP2298743A1 | 1771-EP2298748A2 |
| 1771-EP2298764A1 | 1771-EP2298765A1 | 1771-EP2298768A1 |
| 1771-EP2298772A1 | 1771-EP2298778A1 | 1771-EP2298780A1 |
| 1771-EP2301928A1 | 1771-EP2301933A1 | 1771-EP2305243A1 |
| 1771-EP2305640A2 | 1771-EP2305642A2 | 1771-EP2305660A1 |
| 1771-EP2305668A1 | 1771-EP2305671A1 | 1771-EP2305679A1 |
| 1771-EP2305689A1 | 1771-EP2308833A2 | 1771-EP2308839A1 |
| 1771-EP2308855A1 | 1771-EP2308861A1 | 1771-EP2311453A1 |
| 1771-EP2311807A1 | 1771-EP2311808A1 | 1771-EP2311825A1 |
| 1771-EP2311827A1 | 1771-EP2311829A1 | 1771-EP2311840A1 |
| 1771-EP2311842A2 | 1771-EP2311850A1 | 1771-EP2314590A1 |
| 1771-EP2316459A1 | 1771-EP2316832A1 | 1771-EP2316833A1 |
| 1771-EP2316834A1 | 1771-EP2371811A2 | 1771-EP2374454A1 |
| 1dhi | 1dhj | 2,2'-(9,10-anthracenediylidene)-bis-propanedinitrile |
| 2drc | 4-Amino-10-methylfolic acid | 4-Amino-10-methylfolic acid hydrate |
| 4-Amino-N(sup 10)-methylpteroylglutamic acid | 4-Aminomethylpteroylglutamic acid | 4-amino-N(10)-methylpteroylglutamic acid |
| 4kn0 | 4ocx | 59-05-2 |
| 5947-EP0930075A1 | 5947-EP2269994A1 | 5947-EP2289892A1 |
| 70359-39-6 | 73M731 | A-2190 |
| A-Methopterin | A-Methpterin | A10021 |
| AB02593 | ACN-050905 | ACT03341 |
| AI3-25299 | AKOS016340329 | ANW-72813 |
| AOB5585 | APC-2002 | Abitrexate |
| Abitrexate (Methotrexate) | Amethopterin | Amethopterin L- |
| Amethopterine | An-PDN | Antifolan |
| Antifolan hydrate | BCP13701 | BDBM18050 |
| BDBM66082 | BIDD:PXR0175 | BR-77824 |
| BRD-K59456551-001-09-3 | BRD-K59456551-001-11-9 | Bio1_000486 |
| Bio1_000975 | Bio1_001464 | Brimexate |
| C01937 | C20H22N8O5 | CAS-59-05-2 |
| CCG-35800 | CCRIS 1109 | CHEBI:44185 |
| CHEMBL34259 | CL 14377 | CL-14377 |
| CL14377 | CS-1732 | CTK1G9789 |
| Certified Reference Material | D00142 | DB00563 |
| DL-4-Amino-N10-methylpteroylglutamic acid hydrate | DL-Amethopterin hydrate | DSSTox_CID_822 |
| DSSTox_GSID_20822 | DSSTox_RID_75810 | DTXSID4020822 |
| DivK1c_000114 | EINECS 200-413-8 | EMT 25,299 |
| EU-0100020 | Emtexate | Emthexat |
| Emthexate | FBOZXECLQNJBKD-UHFFFAOYSA-N | FBOZXECLQNJBKD-ZDUSSCGKSA-N |
| FT-0082628 | FT-0601523 | Fauldexato |
| Folex-Pfs | G-301 | GTPL4674 |
| GTPL4815 | Glutamic acid, N-(p-(((2,4-diamino-6-pteridinyl)methyl)methylamino)benzoyl)-, L- | Glutamic acid,4-diamino-6-pteridinyl)methyl] methylamino]benzoyl]-, L-(+)- |
| HMS1568K12 | HMS2095K12 | HMS2233O18 |
| HMS3260C21 | HMS3414L09 | HMS3678L07 |
| HMS3712K12 | HMS500F16 | HSDB 3123 |
| HY-14519 | Hdmtx | IDI1_000114 |
| Intradose-MTX | Intrathecal methotrexate | J10045 |
| KBio1_000114 | KBio2_002338 | KBio2_004906 |
| KBio2_007474 | KBio3_001493 | KBioGR_001172 |
| KBioSS_002341 | KS-00000L8X | KS-5093 |
| KSC269O8T | Kyselina 4-amino-N(sup 10)-methylpteroylglutamova | Kyselina 4-amino-N(sup 10)-methylpteroylglutamova [Czech] |
| Kyselina N-(p-((2,4-diamino-6-pteridinylmethyl)methylamino)benzoyl)-L-glutamova | Kyselina N-(p-((2,4-diamino-6-pteridinylmethyl)methylamino)benzoyl)-L-glutamova [Czech] | L(+)-Amethopterin hydrate |
| L-(+)-N-(p-(((2,4-Diamino-6-pteridinyl)methyl)methylamino)benzoyl)glutamic acid | L-Amethopterin | L-Glutamic acid,4-diamino-6-pteridinyl)methyl]- methylamino]benzoyl]- |
| L-Glutamic acid,N-[4-[[(2,4-diamino-6-pteridinyl)methyl]methylamino]benzoyl]-, hydrate (9CI) | L-Glutamic acid,N-[4-[[(2,4-diamino-6-pteridinyl)methyl]methylamino]benzoyl]-,hydrate(9ci) | L-Glutamicacid, N-[4-[[(2,4-diamino-6-pteridinyl)methyl]methylamino]benzoyl]- |
| LP00020 | LS-249 | Lantarel |
| Lopac0_000020 | Lumexon | MCULE-6523054194 |
| MFCD00150847 | MLS000049968 | MLS001401431 |
| MLS002154208 | MPI-2505 | MPI-5004 |
| MTX | MTX | MTX hydrate |
| Maxtrex | Medsatrexate | Metatrexan |
| Metex | Methoblastin | Methotextrate |
| Methotrexat | Methotrexat-Ebewe | Methotrexate (JP17/USP/INN) |
| Methotrexate - Abitrexate | Methotrexate 1.0 mg/ml in Dimethyl Sulfoxide | Methotrexate [USAN:INN:BAN:JAN] |
| Methotrexate [USAN:USP:INN:BAN:JAN] | Methotrexate for peak identification, European Pharmacopoeia (EP) Reference Standard | Methotrexate for system suitability, European Pharmacopoeia (EP) Reference Standard |
| Methotrexate hydrate | Methotrexate, European Pharmacopoeia (EP) Reference Standard | Methotrexate, L- |
| Methotrexate, PharmaGrade, Manufactured under appropriate controls for use as a raw material in pharma or biopharmaceutical production, meets EP, USP testing specifications | Methotrexate, Pharmaceutical Secondary Standard | Methotrexate, United States Pharmacopeia (USP) Reference Standard |
| Methotrexatum | Methotrexatum [INN-Latin] | Methoxtrexate |
| Methylaminopterin | Methylaminopterin | Methylaminopterin hydrate |
| Methylaminopterinum | Metolate | Metotressato |
| Metotressato [DCIT] | Metotrexato | Metotrexato [INN-Spanish] |
| Metrotex | Mexate-Aq | N-(4-(((2,4-Diamino-6-pteridinyl)methyl)methylamino)benzoyl)-L-glutamicacid |
| N-(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)amino}benzoyl)-L-glutamic acid | N-(p-(((2,4-Diamino-6-pteridinyl)methyl)methylamino)benzoyl)-L-(+)-glutamic acid | N-(p-(((2,4-Diamino-6-pteridyl)methyl)methylamino)benzoyl)glutamic acid |
| N-Bismethylpteroylglutamic acid | N-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)amino}phenyl)carbonyl]-L-glutamic acid | N-[4-[[(2,4-Diamino-6-pteridinyl)methyl] methylamino]benzoyl]-L-glutamic acid |
| N-[4-[[(2,4-diamino-6-pteridinyl)methyl]methylamino]benzoyl]-L-glutamic acid | N-{4-[[(2,4-diaminopteridin-6-yl)methyl](methyl)amino]benzoyl}-L-glutamic acid | NCGC00025060-01 |
| NCGC00025060-02 | NCGC00025060-03 | NCGC00025060-04 |
| NCGC00025060-05 | NCGC00025060-06 | NCGC00025060-07 |
| NCGC00025060-08 | NCGC00025060-09 | NCGC00025060-10 |
| NCGC00025060-11 | NCGC00025060-12 | NCGC00025060-13 |
| NCGC00025060-15 | NCGC00025060-16 | NCGC00254216-01 |
| NCGC00260705-01 | NCI-C04671 | NCIMech_000767 |
| NINDS_000114 | NSC 740 | NSC-740 |
| NSC740 | Nordimet | Novatrex |
| Otrexup | Otrexup (TN) | Prestwick0_000135 |
| Prestwick1_000135 | Prestwick2_000135 | Prestwick_322 |
| Q-201366 | Q422232 | R 9985 |
| RTR-020497 | Rasuvo | Rheumatrex |
| SC-19293 | SCHEMBL12421860 | SCHEMBL3711 |
| SMP2_000020 | SMR000112001 | SMR000449324 |
| SPBio_001094 | SPBio_002149 | SR-01000075682 |
| SR-01000075682-1 | SR-01000075682-2 | SR-01000075682-6 |
| SR-01000597411 | SR-01000597411-1 | STL535338 |
| SW198601-3 | Spectrum2_001077 | Spectrum3_000497 |
| Spectrum4_000616 | Spectrum5_000958 | Spectrum_001836 |
| TCMDC-125858 | Texate | Tocris-1230 |
| Tox21_110944 | Tox21_110944_1 | Tox21_300269 |
| Tox21_500020 | Tremetex | Trexall |
| Trexeron | Trixilem | UNII-99ITO15X8S component FBOZXECLQNJBKD-ZDUSSCGKSA-N |
| UNII-YL5FZ2Y5U1 | W-105347 | W-60383 |
| WLN: T66 BN DN GN JNJ CZ EZ H1N1&R DVMYVQ2VQ | WR-19039 | X 133 |
| Xatmep | Xatmep (TN) | YL5FZ2Y5U1 |
| Z1541638527 | ZINC1529323 | [3H]-methotrexate |
| [3H]methotrexate | cid_126941 | cid_165528 |
| folic acid antagonist | g301 | methotrexate |
| s1210 |
| DrugBank Name | Methotrexate |
| DrugBank | DB00563 |
| CAS Number | 102613-64-9, 133073-73-1, 142155-43-9, 51865-79-3, 59-05-2, 60388-53-6, 70359-39-6 |
| PubChem Compound | 126941 |
| KEGG Compound ID | C01937 |
| KEGG Drug | D00142 |
| PubChem.Substance | 46507678 |
| ChEBI | 44185 |
| PharmGKB | PA450428 |
| ChemSpider | 112728 |
| BindingDB | 66082.0 |
| TTD | DNC000933 |
| Wikipedia | Methotrexate |
| HET | MTX |
| DPD | 5900|6197 |