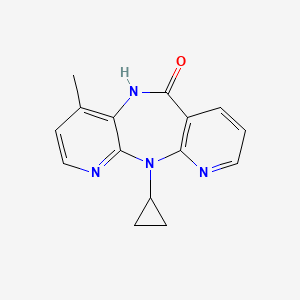
D0090 | Nevirapine
J
J05AR07 Stavudine, lamivudine and nevirapine
[J05AR] Antivirals for treatment of HIV infections, combinations
[J05A] DIRECT ACTING ANTIVIRALS
[J05] ANTIVIRALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
J05AR05 Zidovudine, lamivudine and nevirapine
[J05AR] Antivirals for treatment of HIV infections, combinations
[J05A] DIRECT ACTING ANTIVIRALS
[J05] ANTIVIRALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
J05AG01 Nevirapine
[J05AG] Non-nucleoside reverse transcriptase inhibitors
[J05A] DIRECT ACTING ANTIVIRALS
[J05] ANTIVIRALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Warning |
Aggregated GHS information provided by 23 companies from 4 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H302 (17.39%): Harmful if swallowed [Warning Acute toxicity, oral] H412 (95.65%): Harmful to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P264, P270, P273, P301+P312, P330, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LDLo | oral | 400mg/kg (400mg/kg) | Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 41, Pg. 2209, 1999. | |
| dog | LD | oral | > 3200mg/kg (3200mg/kg) | Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 41, Pg. 2209, 1999. | |
| 11-CYCLOPROPYL-4-METHYL-5H-DIPYRIDO[3,2-B:2',3'-E][1,4]DIAZEPIN-6(11H)-ONE | 11-CYCLOPROPYL-5,11-DIHYDRO-4-METHYL-6H-DIPYRIDO[3,2-B:2',3'-E][1,4]DIAZEPIN-6-ONE | 11-Cyclopropyl-4-methyl-5,11-dihydro-6H-dipyrido[2,3-e:3',2'-b][1,4]diazepin-6-one |
| 11-Cyclopropyl-4-methyl-5,11-dihydro-6H-dipyrido[2,3-e:3',2'-b][1,4]diazepin-6-one & PRO 140 (Anti-CCR5 monoclonal antibody) | 11-Cyclopropyl-4-methyl-5,11-dihydro-6H-dipyrido[3,2-b:2 inverted exclamation mark ,3 inverted exclamation mark -e][1,4]diazepin-6-one | 11-Cyclopropyl-4-methyl-5,11-dihydro-6H-dipyrido[3,2-b:2',3'-e][1,4]diazepin-6-one |
| 11-Cyclopropyl-4-methyl-5H-dipyrido[3,2-b | 11-Cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido(3,2-b:2',3'-e)(1,4)diazepin-6-one | 11-Cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3,2-b:2', 3'-e][1,4]diazepin-6-one |
| 11-cyclopropyl-4-methyl-5H-dipyrido[2,3-b:3',2'-e][1,4]diazepin-6-one | 11-cyclopropyl-4-methyl-5H-dipyrido[[?],[?]][1,4]diazepin-6-one | 11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido-[3,2-b:2',3'-e][1,4]diazepin-6-one |
| 11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3,2-b :2',3'-e][1,4 ]diazepin-6-one | 11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3,2-b:2 ,3 -e][1,4]diazepin-6-one | 129618-40-2 |
| 1vrt | 2-cyclopropyl-7-methyl-2,4,9,15-tetraazatricyclo[9.4.0.0(3),]pentadeca-1(15),3(8),4,6,11,13-hexaen-10-one | 2-cyclopropyl-7-methyl-2,4,9,15-tetraazatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3,5,7,12,14-hexaen-10-one |
| 2-cyclopropyl-7-methyl-2,4,9,15-tetraazatricyclo[9.4.0.0^{3,8}]pentadeca-1(15),3,5,7,11,13-hexaen-10-one | 2hny | 5H-Dipyrido(3,2-b:2',3'-e)(1,4)diazepin-6-one, 5,11-dihydro-11-cyclopropyl-4-methyl- |
| 618N402 | 6H-Dipyrido(3,2-b:2',3'-e)(1,4)diazepin-6-one, 11-cyclopropyl-5,11-dihydro-4-methyl- | 6H-dipyrido[2,3-e:3',2'-b][1,4]diazepin-6-one, 11-cyclopropyl-5,11-dihydro-4-methyl- |
| 99DK7FVK1H | AB0013383 | AB00393001-13 |
| AB00393001-15 | AB00393001_16 | AB00393001_17 |
| AB07544 | AB2000266 | AC-643 |
| AK143126 | AKOS005504351 | ALBB-027264 |
| BBL010768 | BCP05587 | BDBM1434 |
| BI-RG 587 | BI-RG 587;NSC 641530;NVP | BI-RG-587 |
| BI-RG-587 | BI-RG-587 & CD4-IgG | BIDD:GT0326 |
| BIRG 0587 | BIRG 0587 | BIRG 587 |
| BIRG-0587 | BIRG-587 | BIRG587 |
| BIRG587 | C07263 | C15H14N4O |
| CAS-129618-40-2 | CCG-100939 | CHEBI:63613 |
| CHEMBL57 | CPD000048458 | CS-2252 |
| CTK8E7455 | Certified Reference Material | D00435 |
| DB-041930 | DB00238 | DSSTox_CID_10787 |
| DSSTox_GSID_31797 | DSSTox_RID_78889 | DTXSID7031797 |
| F2173-0607 | FT-0607215 | H954 |
| HMS2051J09 | HMS2231O23 | HMS3264D21 |
| HMS3371E03 | HMS3393J09 | HMS3655I08 |
| HMS3715B10 | HSDB 7164 | HSDB 7164 |
| HY-10570 | KS-00000KZF | KS-5019 |
| LS-2289 | MCULE-8608154492 | MFCD00866928 |
| MFCD00866928 (98%) | MLS000084585 | MLS000759409 |
| MLS001055309 | MLS001201730 | MLS001424058 |
| MLS006011423 | N0922 | N11-Cyclopropyl-4-methyl-5,11-dihydro-6H-dipyrido[3,2-b:2',3'-e]-[1,4]diazepin-6-one & CD4-immunoadhesin |
| NC00189 | NCGC00065890-02 | NCGC00065890-03 |
| NCGC00065890-04 | NCGC00065890-05 | NCGC00065890-07 |
| NCGC00258324-01 | NEV | NON-NUCLEOSIDE RT INHIBITOR NEVIRAPINE |
| NQDJXKOVJZTUJA-UHFFFAOYSA-N | NSC 641530 | NSC 641530 |
| NSC-641530 | NSC-759902 | NSC641530 |
| NSC759902 | NVP | NVP |
| Nevirapine & CD4-IgG | Nevirapine & PRO 140 | Nevirapine (JAN/USP/INN) |
| Nevirapine (Viramune) | Nevirapine (anhydrous), European Pharmacopoeia (EP) Reference Standard | Nevirapine [USAN:INN] |
| Nevirapine [USAN:USP:INN:BAN] | Nevirapine anhydrous | Nevirapine anhydrous, United States Pharmacopeia (USP) Reference Standard |
| Nevirapine for peak identification, European Pharmacopoeia (EP) Reference Standard | Nevirapine solution, 1.0 mg/mL in methanol, certified reference material | Nevirapine, 98% |
| Nevirapine, Pharmaceutical Secondary Standard | Nevirapine,(S) | Opera_ID_934 |
| Pharmakon1600-01503842 | Q263713 | R3924 |
| RT-014690 | S1742 | SAM001246551 |
| SC-17266 | SCHEMBL3318 | SMR000048458 |
| ST24038235 | STK580320 | SW197569-2 |
| SY009679 | Tox21 110982 | Tox21_110982 |
| Tox21_110982_1 | Tox21_200770 | UNII-99DK7FVK1H |
| Viramune | Viramune (TN) | Viramune IR |
| Viramune XR | Viramune(TM) | Viramune;BI-RG 587 |
| Z1695906730 | ZINC4778 | nevirapine |
| DrugBank Name | Nevirapine |
| DrugBank | DB00238 |
| CAS Number | 129618-40-2 |
| PubChem Compound | 4463 |
| KEGG Compound ID | C07263 |
| KEGG Drug | D00435 |
| PubChem.Substance | 46506789 |
| ChEBI | 63613 |
| PharmGKB | PA450616 |
| ChemSpider | 4308 |
| BindingDB | 1434.0 |
| TTD | DAP000184 |
| Wikipedia | Nevirapine |
| HET | NVP |
| DPD | 11796 |