Drug
D0095 | Pergolide
N
N04BC02 Pergolide
[N04BC] Dopamine agonists
[N04B] DOPAMINERGIC AGENTS
[N04] ANTI-PARKINSON DRUGS
[N] Nervous system
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Danger |
The GHS information provided by 1 company from 1 notification to the ECHA C&L Inventory. H300 (100%): Fatal if swallowed [Danger Acute toxicity, oral] H351 (100%): Suspected of causing cancer [Warning Carcinogenicity] H361 (100%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] |
P201, P202, P264, P270, P281, P301+P310, P308+P313, P321, P330, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
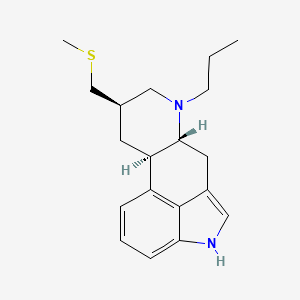
| (2R,4R,7R)-4-[(methylsulfanyl)methyl]-6-propyl-6,11-diazatetracyclo[7.6.1.0^{2,7}.0^{12,16}]hexadeca-1(16),9,12,14-tetraene | (6aR,9R,10aR)-9-(Methylthiomethyl)-7-propyl-4,6,6a,7,8,9,10,10a-octahydroindolo[4,3-fg]quinoline | (6aR,9R,10aR)-9-Methylsulfanylmethyl-7-propyl-4,6,6a,7,8,9,10,10a-octahydro-indolo[4,3-fg]quinolin-7-ium |
| (6aR,9R,10aR)-9-Methylsulfanylmethyl-7-propyl-4,6,6a,7,8,9,10,10a-octahydro-indolo[4,3-fg]quinoline | (8beta)-8-[(methylsulfanyl)methyl]-6-propylergoline | (8beta)-8-[(methylthio)methyl]-6-propylergoline |
| 104P221 | 24MJ822NZ9 | 5-Bromo-7-methyl-4,6,6a,7,8,9-hexahydro-indolo[4,3-fg]quinoline-9-carboxylic acid (10b-hydroxy-5-isobutyl-2-isopropyl-3,6-dioxo-octahydro-oxazolo[3,2-a]pyrrolo[2,1-c]pyrazin-2-yl)-amide |
| 66104-22-1 | 9-Methylsulfanylmethyl-7-propyl-4,6,6a,7,8,9,10,10a-octahydro-indolo[4,3-fg]quinolin-7-ium(Pergolide) | 9-Methylsulfanylmethyl-7-propyl-4,6,6a,7,8,9,10,10a-octahydro-indolo[4,3-fg]quinoline |
| 9-Methylsulfanylmethyl-7-propyl-4,6,6a,7,8,9,10,10a-octahydro-indolo[4,3-fg]quinoline | 9-Methylsulfanylmethyl-7-propyl-4,6,6a,7,8,9,10,10a-octahydro-indolo[4,3-fg]quinoline(pergolide) | 9-Methylsulfanylmethyl-7-propyl-4,6,6a,7,8,9,10,10a-octahydro-indolo[4,3-fg]quinoline, mesylate (Pergolide) |
| AB00053740-13 | AB00053740_14 | AB00053740_15 |
| BCP18331 | BDBM50017543 | BDBM50028421 |
| BIDD:GT0177 | BPBio1_000254 | BPBio1_001211 |
| BRD-K60770992-001-01-8 | BRD-K60770992-066-05-2 | BRD-K60770992-066-15-1 |
| BSPBio_000230 | BSPBio_003156 | Biomol-NT_000025 |
| C-19410 | C07425 | CC-33590 |
| CCG-205064 | CHEBI:63617 | CHEMBL1275 |
| CHEMBL531 | D-8beta-((Methylthio)methyl)-6-propylergoline | D08339 |
| DB01186 | DTXSID2023438 | DivK1c_000442 |
| Ergoline, 8-((methylthio)methyl)-6-propyl-, (8beta)- | GTPL48 | HMS2089C18 |
| IDI1_000442 | InChI=1/C19H26N2S/c1-3-7-21-11-13(12-22-2)8-16-15-5-4-6-17-19(15)14(10-20-17)9-18(16)21/h4-6,10,13,16,18,20H,3,7-9,11-12H2,1-2H3/t13-,16-,18-/m1/s | KBio1_000442 |
| KBio2_002127 | KBio2_004695 | KBio2_007263 |
| KBio3_002656 | KBioGR_001409 | KBioSS_002127 |
| LS-64479 | LY 141B | LY-127,809 |
| LY-127809 | Lopac0_000984 | NCGC00017366-02 |
| NCGC00017366-03 | NCGC00017366-04 | NCGC00017366-05 |
| NCGC00017366-06 | NCGC00017366-10 | NCGC00142538-01 |
| NCGC00142538-02 | NCGC00142538-03 | NINDS_000442 |
| P2200 | Pergolida | Pergolida [INN-Spanish] |
| Pergolide (INN) | Pergolide [INN:BAN] | Pergolidum |
| Pergolidum [INN-Latin] | Permax | Permax (TN) |
| Prestwick0_000295 | Prestwick1_000295 | Prestwick2_000295 |
| Prestwick3_000295 | Q415752 | SBI-0050957.P003 |
| SCHEMBL26921 | SPBio_002099 | SPBio_002449 |
| SR-01000721840 | SR-01000721840-8 | ST057534 |
| Spectrum2_001970 | Spectrum3_001588 | Spectrum4_000835 |
| Spectrum5_001649 | Spectrum_001647 | TNP00315 |
| UNII-24MJ822NZ9 | YEHCICAEULNIGD-MZMPZRCHSA-N | ZINC3786466 |
| [2-(1H-Indol-4-yl)-ethyl]-methyl-amine | cid_47811 | compound with methanesulfonic acid |
| pergolide |
1. Dykens et al. (2007)