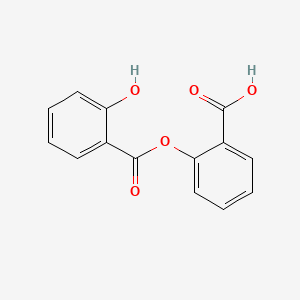
D0110 | Salsalate
N
N02BA06 Salsalate
[N02BA] Salicylic acid and derivatives
[N02B] OTHER ANALGESICS AND ANTIPYRETICS
[N02] ANALGESICS
[N] Nervous system
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Warning |
Aggregated GHS information provided by 45 companies from 5 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H302 (88.89%): Harmful if swallowed [Warning Acute toxicity, oral] H315 (11.11%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (11.11%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H400 (84.44%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P264, P270, P273, P280, P301+P312, P302+P352, P305+P351+P338, P321, P330, P332+P313, P337+P313, P362, P391, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| 2-((2-Hydroxybenzoyl)oxy)benzoic acid | 2-(2-hydroxybenzoyl)oxybenzoic acid | 2-(2-hydroxybenzoyloxy)benzoic acid |
| 2-(2-hydroxyphenylcarbonyloxy)benzoic acid | 2-Carboxyphenyl salicylate | 2-Carboxyphenyl salicylate, AldrichCPR |
| 2-Hydroxybenzoic Acid 2-Carboxyphenyl Ester | 2-Hydroxybenzoic Acid 2-Carboxyphenyl Ester | 2-Hydroxybenzoic acid 2-carboxyphenyl ester |
| 2-Salicyloyloxybenzoic Acid | 2-Salicylsalicylic acid | 2-[(2-Hydroxybenzoyl)oxy]benzoic Acid (Salicylsalicylic Acid) |
| 2-[(2-Hydroxybenzoyl)oxy]benzoic acid # | 2-[(2-hydroxyphenyl)carbonyloxy]benzoic acid | 2-{[(2-hydroxyphenyl)carbonyl]oxy}benzoic acid |
| 552-94-3 | AB01563259_01 | AB01563259_02 |
| AC-18298 | ACMC-209lm5 | AK114551 |
| AKOS003368478 | ANW-32283 | API0002421 |
| AS-12645 | AX8015186 | Aspirin Impurity E |
| BDBM85244 | BRN 2590908 | BSPBio_001665 |
| Benzoic acid, 2-carboxyphenyl ester | Benzoic acid, 2-hydroxy-, 2-carboxyphenyl ester | C-16063 |
| CAS-552-94-3 | CAS_552-94-3 | CC-09787 |
| CCG-39652 | CHEBI:9014 | CHEMBL154111 |
| CS-4891 | Certified Reference Material | D00428 |
| DB-020760 | DB01399 | DSSTox_CID_3572 |
| DSSTox_GSID_23572 | DSSTox_RID_77088 | DTXSID1023572 |
| Diacesal | Diplosal | Disalcid |
| Disalcid (TN) | Disalgesic | Disalicylic acid |
| Disalicylic acid; | Disalicylsaeure | Disalyl |
| EINECS 209-027-4 | FT-0632376 | GL4615 |
| HMS2091A05 | HMS3652P07 | HY-B1245 |
| KBio2_002563 | KBio2_005131 | KBio2_007699 |
| KBio3_001165 | KBioGR_001500 | KBioSS_002572 |
| KS-00000WSM | LS-37546 | MCULE-7200376848 |
| MFCD00020252 | Mono-gesic | NCGC00096014-01 |
| NCGC00096014-02 | NCGC00096014-03 | NSC 49171 |
| NSC-49171 | NSC-755823 | NSC49171 |
| NSC755823 | NSC_5161 | Nobacid |
| O-Salicylcylsalicylsaeure | O-Salicyloylsalicylic Acid | Pharmakon1600-00200331 |
| Q-100630 | Q1320691 | S0129 |
| SBB003097 | SBI-0206687.P002 | SCHEMBL15562 |
| SPBio_000845 | SPECTRUM200331 | SR-05000001536 |
| SR-05000001536-1 | ST083489 | ST24027407 |
| SW219189-1 | Sal Ester Sal | Salflex |
| Salical | Salicylic Acid 2-Carboxyphenyl Ester | Salicylic Acid Salicylate |
| Salicylic acid bimolecular ester | Salicylic acid, bimolecular ester | Salicylic acid, salicylate |
| Salicyloxysalicylic acid | Salicyloylsalicylic acid | Salicylsalicylic Acid (2-[(2-Hydroxybenzoyl)oxy]benzoic Acid) |
| Salicylsalicylic acid | Salicylsalicylic acid, 98% | Salicylsalicylic acid;Disalicylic acid |
| Salina | Saloxium | Salsalate |
| Salsalate (Aspirin Impurity E), Pharmaceutical Secondary Standard | Salsalate (USP/INN) | Salsalate [USAN:INN:BAN] |
| Salsalate [USAN:USP:INN:BAN] | Salsalate, >=98% (HPLC) | Salsalate, United States Pharmacopeia (USP) Reference Standard |
| Salsalato | Salsalato [INN-Spanish] | Salsalatum |
| Salsalatum [INN-Latin] | Salysal | Sasapirin |
| Sasapyrin | Sasapyrine (JAN) | Sasapyrinum |
| Spectrum2_000693 | Spectrum3_000173 | Spectrum4_000940 |
| Spectrum5_000670 | Spectrum_001998 | TC-121028 |
| Tox21_111548 | Tox21_111548_1 | UNII-V9MO595C9I |
| V9MO595C9I | WVYADZUPLLSGPU-UHFFFAOYSA-N | ZINC2062 |
| o-Salicylsalicylic acid | s4188 | sasapyrine |