Drug
D0270 | Valinomycin
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| TRANSPORT OF CHARGE BUT NOT PROTONS | affect | 201 | ||||||
| GLUCOSE GALACTOSE IC50 RATIO | 1 | LUHMES (Lund human mesencephalic) cells | Glc–Gal–NeuriTox assay | Negative | EC25(NA) [Glc/Gal] | 326 | ||
| MITOCHONDRIAL MOTILITY | 10 μM | decrease | 217 | |||||
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Danger |
Aggregated GHS information provided by 44 companies from 4 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H300 (100%): Fatal if swallowed [Danger Acute toxicity, oral] H310 (100%): Fatal in contact with skin [Danger Acute toxicity, dermal] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P262, P264, P270, P280, P301+P310, P302+P350, P310, P321, P322, P330, P361, P363, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| rabbit | LD50 | skin | 5mg/kg (5mg/kg) | Drug and Chemical Toxicology. Vol. 8, Pg. 451, 1985. | |
| rat | LD50 | intraperitoneal | 800ug/kg (0.8mg/kg) | Drug and Chemical Toxicology. Vol. 8, Pg. 451, 1985. | |
| mouse | LD50 | subcutaneous | 4140ug/kg (4.14mg/kg) | Antibiotics and Chemotherapy Vol. 12, Pg. 482, 1962. | |
| mouse | LD50 | oral | 2500ug/kg (2.5mg/kg) | "Antibiotics: Origin, Nature, and Properties," Korzyoski, T., et al., eds., Washington, DC, American Soc. for Microbiology, 1978Vol. 1, Pg. 325, 1978. | |
| mouse | LD50 | intraperitoneal | 390ug/kg (0.39mg/kg) | National Cancer Institute Screening Program Data Summary, Developmental Therapeutics Program. Vol. JAN1986, | |
| mouse | LD50 | intravenous | 180ug/kg (0.18mg/kg) | Cancer Research. Vol. 46, Pg. 5518, 1986. | |
| rat | LD50 | oral | 4mg/kg (4mg/kg) | Drug and Chemical Toxicology. Vol. 8, Pg. 451, 1985. | |
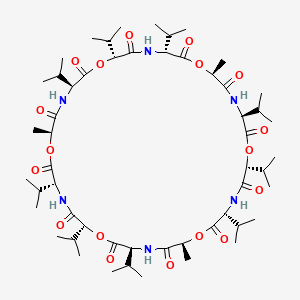
| (3R,6R,9S,12S,15R,18R,21S,24S,27R,30R,33S,36S)-3,6,9,15,18,21,27,30,33-nonaisopropyl-12,24,36-trimethyl-1,7,13,19,25,31-hexaoxa-4,10,16,22,28,34-hexaazacyclohexatriacontane-2,5,8,11,14,17,20,23,26,29,32,35-dodecone | (3R,6R,9S,12S,15R,18R,21S,24S,27R,30R,33S,36S)-3,6,9,15,18,21,27,30,33-nonaisopropyl-12,24,36-trimethyl-1,7,13,19,25,31-hexaoxa-4,10,16,22,28,34-hexazacyclohexatriacontane-2,5,8,11,14,17,20,23,26,29,32,35-dodecone | 1,7,13,19,25,31-Hexaoxa-4,10,16,22,28,34-hexaazacyclohexatriacontane- 2,5,8,11,14,17,20,23,26,29,32,35-dodecone, 12,24,36-trimetyl-3,6,9,15,18,21,27,30,33-nonakis(1-methylethyl)- |
| 2001-95-8 | 4-27-00-09728 (Beilstein Handbook Reference) | AKOS024457589 |
| Antibiotic N-329 B | BDBM50237619 | BRN 0078657 |
| CCG-208283 | CHEBI:28545 | CHEMBL223643 |
| CS-0029275 | Cyclic(D-.alpha.-hydroxyisovaleryl-D-valyl-L-lactoyl-L-valyl-D-.alpha.-hydroxyisovaleryl-D-valyl-L-lactoyl-L-valyl-D-.alpha.-hydroxyisovaleryl-D-valyl-L-lactoyl-L-valyl ) | Cyclic(D-alpha-hydroxyisovaleryl-D-valyl-L-lactoyl-L-valyl-D-alpha-hydroxyisovaleryl-D-valyl-L-lactoyl-L-valyl-D-alpha-hydroxyisovaleryl-D-valyl-L-lactoyl-L-valyl) |
| Cyclo(D-?-hydroxyisovaleryl-D-valyl-L-lactoyl-L-valyl-D-?-hydroxyisovaleryl-D-valyl-L-lactoyl-L-valyl-D-?-hydroxyisovaleryl-D-valyl-L-lactoyl-L-valyl) | EINECS 217-896-6 | HSDB 6423 |
| HY-N6693 | N561YS75MN | NSC 122023 |
| NSC122023 | Potassium ionophore I | Potassium ionophore I - cocktail B, Selectophore(TM) |
| Q417504 | SR-05000002323 | SR-05000002323-2 |
| UNII-N561YS75MN | Valinomicin | balinomycin |
| cyclo[-D-O-Val-D-Val-L-O-Ala-L-Val]3 | valino | valinomycin |
| DrugBank Name | Valinomycin |
| DrugBank | DB14057 |
| CAS Number | 2001-95-8 |
| PubChem Compound | 3000706 |
| KEGG Compound ID | C06684 |
| ChEBI | 28545 |
| PharmGKB | PA151917011 |
| ChemSpider | 21493802 |
| BindingDB | 50237619.0 |
| Wikipedia | Valinomycin |