D0285 | Acetylcysteine
S
V
R
V03AB23 Acetylcysteine
[V03AB] Antidotes
[V03A] ALL OTHER THERAPEUTIC PRODUCTS
[V03] ALL OTHER THERAPEUTIC PRODUCTS
[V] Various ATC structures
S01XA08 Acetylcysteine
[S01XA] Other ophthalmologicals
[S01X] OTHER OPHTHALMOLOGICALS
[S01] OPHTHALMOLOGICALS
[S] Sensory organs
R05CB01 Acetylcysteine
[R05CB] Mucolytics
[R05C] EXPECTORANTS, EXCL. COMBINATIONS WITH COUGH SUPPRESSANTS
[R05] COUGH AND COLD PREPARATIONS
[R] Respiratory system
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| RSS PRODUCTION | rho-GFP (Redox-sensitive GFP) | 244 | ||||||
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Warning |
Aggregated GHS information provided by 51 companies from 8 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. Reported as not meeting GHS hazard criteria by 26 of 51 companies. For more detailed information, please visit ECHA C&L website Of the 7 notification(s) provided by 25 of 51 companies with hazard statement code(s): H315 (64%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (96%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (60%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 4400mg/kg (4400mg/kg) | European Patent Application. Vol. #0192191, | |
| mouse | LD50 | intraperitoneal | 400mg/kg (400mg/kg) | National Technical Information Service. Vol. AD691-490, | |
| dog | LD50 | intravenous | 700mg/kg (700mg/kg) | Pharmacological and Biochemical Properties of Drug Substances. Vol. 2, Pg. 479, 1979. | |
| dog | LD50 | oral | > 1gm/kg (1000mg/kg) | Pharmacological and Biochemical Properties of Drug Substances. Vol. 2, Pg. 479, 1979. | |
| dog | LD50 | intraperitoneal | 700mg/kg (700mg/kg) | Pharmacological and Biochemical Properties of Drug Substances. Vol. 2, Pg. 479, 1979. | |
| mouse | LD50 | intravenous | 3800mg/kg (3800mg/kg) | Journal of Medicinal Chemistry. Vol. 10, Pg. 1172, 1967. | |
| rat | LD50 | oral | 5050mg/kg (5050mg/kg) | Toxicology and Applied Pharmacology. Vol. 18, Pg. 185, 1971. | |
| child | TDLo | multiple routes | 8480mg/kg/3D- (8480mg/kg) | liver: liver function tests impaired | Pediatrics. Vol. 79, Pg. 281, 1987. |
| rat | LD50 | intravenous | 1140mg/kg (1140mg/kg) | gastrointestinal: other changes | European Journal of Respiratory Diseases. Vol. 61(Suppl, |
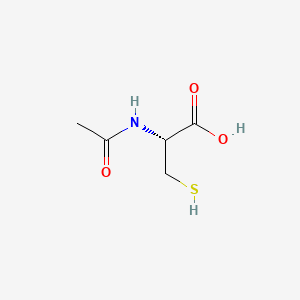
| (2R)-2-(acetylamino)-3-mercaptopropanoic acid | (2R)-2-(acetylamino)-3-sulfanylpropanoic acid | (2R)-2-Acetamido-3-sulfanyl-propanoic acid |
| (2R)-2-acetamido-3-sulfanylpropanoic acid | (2R)-2-acetylamino-3-mercapto-propionic acid | (2R)-2-acetylamino-3-sulfanylpropanoic acid |
| (R)-2-Acetamido-3-mercaptopropanoic acid | (R)-2-acetylamino-3-mercaptopropanoic acid | (R)-mercapturic acid |
| (S)-2-Acetamido-3-mercaptopropanoic acid | 16A911 | 2-Acetylamino-3-mercapto-propionate |
| 2-Acetylamino-3-mercapto-propionic acid | 616-91-1 | 66233-EP2298305A1 |
| 66233-EP2308851A1 | A 7250 | A-1100 |
| A0905 | AB0013800 | AB00382974-12 |
| AB00382974_13 | AB1002786 | AC-16071 |
| AC-24117 | AKOS015841009 | AM20100502 |
| ANTIOXIDANT MODEL (TRAMP) -N-ACETYLCYSTEINE | ANW-33915 | Ac-Cys-OH |
| AcCys | Acetadote | Acetein |
| Acetilcisteina | Acetilcisteina [INN-Spanish] | Acetyl Cysteine,(S) |
| Acetyl-L-cysteine | Acetylcysteine (JP17/USP/INN) | Acetylcysteine [USAN:INN:BAN] |
| Acetylcysteine [USAN:USP:INN:BAN:JAN] | Acetylcysteine, British Pharmacopoeia (BP) Reference Standard | Acetylcysteine, European Pharmacopoeia (EP) Reference Standard |
| Acetylcysteine, United States Pharmacopeia (USP) Reference Standard | Acetylcysteinum | Acetylcysteinum [INN-Latin] |
| Airbron | BDBM50420190 | BP-12854 |
| BR-46577 | BRD-K59058747-001-20-9 | BSPBio_001794 |
| Broncholysin | Brunac | C06809 |
| CABC898A-E48B-4E13-9F72-98D0609A1854 | CAS-616-91-1 | CCG-204176 |
| CCRIS 3764 | CHEBI:28939 | CHEMBL600 |
| CS-2160 | CTK2F2887 | Certified Reference Material |
| Cetylev | Cysteine, N-acetyl-, L- | D00221 |
| DB-038288 | DB06151 | DSSTox_CID_21 |
| DSSTox_GSID_20021 | DSSTox_RID_75324 | DTXSID5020021 |
| EINECS 210-498-3 | EN300-72028 | EU-0100081 |
| Exomuc | F1905-7178 | FT-0629832 |
| Fabrol | Fluatox | Fluimicil Infantil |
| Fluimucetin | Fluimucil | Flumil |
| Flumucetin | Fluprowit | GS-3121 |
| HMS2234J22 | HMS3260A04 | HMS3655G11 |
| HMS3715D03 | HSDB 3003 | HY-B0215 |
| I630 | Ilube | Inspir |
| J-507685 | KBio3_001294 | KBioGR_000554 |
| KS-0000026W | KSC352Q8P | L-.alpha.-Acetamido-.beta.-mercaptopropionic acid |
| L-Acetylcysteine | L-Cysteine, N-acetyl- | L-Cysteine, N-acetyl- & Tumor necrosis factor |
| L-alpha-Acetamido-beta-mercaptopropionic acid | LNAC | LNAC |
| LP00081 | LS-2165 | Lopac0_000081 |
| Lysomucil | MFCD00004880 | MLS000028419 |
| MLS001076125 | MLS006011563 | MUCOMYST (TN) |
| MUCOSIL-10 | MUCOSIL-20 | Mercapturic acid |
| Mercapturic acid, (R)- | Muco sanigen | Mucocedyl |
| Mucofilin | Mucolator | Mucolyticum |
| Mucolyticum Lappe | Mucolyticum-Lappe | Mucolytikum Lappe |
| Mucomyst | Mucosil | Mucosolvin |
| Mucret | N-Acety-L-Cysteine | N-Acetyl cysteine |
| N-Acetyl-3-mercaptoalanine | N-Acetyl-L-(+)-cysteine | N-Acetyl-L-cysteine |
| N-Acetyl-L-cysteine & Tumor necrosis factor (TNF) | N-Acetyl-L-cysteine hydrochloride | N-Acetyl-L-cysteine, 98% |
| N-Acetyl-L-cysteine, BioXtra, >=99% (TLC) | N-Acetyl-L-cysteine, PharmaGrade, Ajinomoto, Manufactured under appropriate GMP controls for pharma or biopharmaceutical production, suitable for cell culture | N-Acetyl-L-cysteine, Pharmaceutical Secondary Standard |
| N-Acetyl-L-cysteine, SAJ special grade, 98.0-102.0% | N-Acetyl-L-cysteine, Sigma Grade, >=99% (TLC), powder | N-Acetyl-L-cysteine, Vetec(TM) reagent grade, 98% |
| N-Acetyl-L-cysteine, cell culture tested, BioReagent | N-Acetyl-cysteine | N-Acetylcysteine |
| N-acetyl-(R)-cysteine | N-acetyl-L-cystein | N-acetyl-l-cys |
| N-acetylcystein | N-alpha-Acetyl-L-cysteine | NAC |
| NAC & TNF | NAC,L-alpha-Acetamido-beta-mercaptopropionic acid | NAC-TB |
| NCGC00015086-04 | NCGC00022304-03 | NCGC00022304-04 |
| NCGC00022304-05 | NCGC00022304-06 | NCGC00022304-07 |
| NCGC00022304-08 | NCGC00258631-01 | NCGC00260766-01 |
| NSC 111180 | NSC-111180 | NSC111180 |
| Naxid (Salt/Mix) | Neo-fluimucil | Opera_ID_452 |
| PWKSKIMOESPYIA-BYPYZUCNSA-N | Parvolex | PubChem12963 |
| Q375613 | RK-0202 | RTC-066653 |
| Respaire | SC-09436 | SC2 |
| SCHEMBL5292 | SMR000058377 | SPBio_000012 |
| SR-01000075439 | SR-01000075439-1 | SR-01000075439-3 |
| SR-01000075439-5 | ST2408139 | ST50824849 |
| SW199597-2 | Sodium 2-acetamido-3-mercaptopropionate | Spectrum2_000086 |
| Spectrum3_000287 | Spectrum4_000137 | Spectrum5_000764 |
| Syntemucol | TC-066653 | Tixair |
| Tox21_110877 | Tox21_110877_1 | Tox21_201078 |
| Tox21_500081 | UNII-2SPH1IMO2V component PWKSKIMOESPYIA-BYPYZUCNSA-N | UNII-WYQ7N0BPYC |
| WYQ7N0BPYC | ZINC3589203 | acetyl cysteine |
| acetylcysteine | acetylcysteine (n-acetyl-l-cysteine) | component of Naxid |
| cysteine, N-acetyl- | mercapturic acid | s1623 |
| DrugBank Name | Acetylcysteine |
| DrugBank | DB06151 |
| CAS Number | 18829-79-3, 19542-74-6, 38520-57-9, 616-91-1, 7218-04-4 |
| PubChem Compound | 12035 |
| KEGG Compound ID | C06809 |
| KEGG Drug | D00221 |
| PubChem.Substance | 99443235 |
| ChEBI | 28939 |
| PharmGKB | PA448033 |
| ChemSpider | 11540 |
| BindingDB | 50420190.0 |
| TTD | DNC000981 |
| Wikipedia | Acetylcysteine |
| HET | SC2 |
| DPD | 10303 |