| 120899-48-1 |
12227-89-3 |
DB06215 |
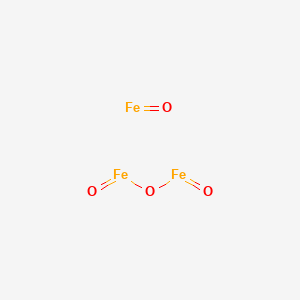
| Fe3O4 |
IRON PIGMENT BLACK (E172) |
Iron (II,III) oxide |
| Iron Oxide Black |
Iron oxide(II,III), magnetic nanoparticles solution, 10 nm avg. part. size (TEM), PEG functionalized, 1 mg/mL Fe in H2O, dispersion |
Iron oxide(II,III), magnetic nanoparticles solution, 10 nm avg. part. size (TEM), amine functionalized, 1 mg/mL Fe in H2O, dispersion |
| Iron oxide(II,III), magnetic nanoparticles solution, 10 nm avg. part. size (TEM), carboxylic acid functionalized, 5 mg/mL Fe in H2O, dispersion |
Iron oxide(II,III), magnetic nanoparticles solution, 10 nm avg. part. size, 5 mg/mL in H2O |
Iron oxide(II,III), magnetic nanoparticles solution, 10 nm avg. part. size, 5 mg/mL in toluene |
| Iron oxide(II,III), magnetic nanoparticles solution, 10 nm diameter, biotin functionalized, 1 mg/mL Fe, dispersion in H2O |
Iron oxide(II,III), magnetic nanoparticles solution, 20 nm avg. part. size, 5 mg/mL in H2O |
Iron oxide(II,III), magnetic nanoparticles solution, 20 nm avg. part. size, 5 mg/mL in toluene |
| Iron oxide(II,III), magnetic nanoparticles solution, 30 nm avg. part. size (TEM), PEG functionalized, dispersion, 1 mg/mL Fe in H2O |
Iron oxide(II,III), magnetic nanoparticles solution, 30 nm avg. part. size (TEM), amine functionalized, 1 mg/mL Fe in H2O, dispersion |
Iron oxide(II,III), magnetic nanoparticles solution, 5 nm avg. part. size (TEM), PEG functionalized, 1 mg/mL Fe in H2O, dispersion |
| Iron oxide(II,III), magnetic nanoparticles solution, 5 nm avg. part. size (TEM), amine functionalized, 1 mg/mL Fe in H2O, dispersion |
Iron oxide(II,III), magnetic nanoparticles solution, 5 nm avg. part. size (TEM), biotin functionalized, 1 mg/mL Fe in H2O, dispersion |
Iron oxide(II,III), magnetic nanoparticles solution, 5 nm avg. part. size, 5 mg/mL in H2O |
| Iron oxide(II,III), magnetic nanoparticles solution, 5 nm avg. part. size, 5 mg/mL in toluene |
Iron(II,III) oxide, 99.99% trace metals basis |
Iron(II,III) oxide, CP |
| Iron(II,III) oxide, nanopowder, 50-100 nm particle size (SEM), 97% trace metals basis |
Iron(II,III) oxide, powder, <5 mum, 95% |
Iron(II,III)oxide |
| Q411235 |
S350 |
SZVJSHCCFOBDDC-UHFFFAOYSA-N |