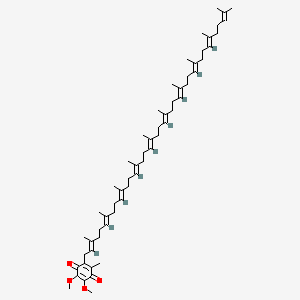
Drug
D0412 | Ubidecarenone
C
C01EB09 Ubidecarenone
[C01EB] Other cardiac preparations
[C01E] OTHER CARDIAC PREPARATIONS
[C01] CARDIAC THERAPY
[C] Cardiovascular system
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| ANTIOXIDANT PRODUCTION | 248 | |||||||
| a 1,4-benzoquinone | a component of the electron transport chain | powerful antioxidant |
| powerful antioxidant that removes free radicals, inhibits the initiation and propagation of lipid peroxidation in cellular biomembranes, and aids in the regeneration of alpha-tocopherol |
| DrugBank Name | Ubidecarenone |
| DrugBank | DB09270 |
| CAS Number | 1339-63-5, 134887-79-9, 137234-88-9, 17745-45-8, 27696-12-4, 303-98-0 |
| PubChem Compound | 5281915 |
| KEGG Compound ID | C11378 |
| KEGG Drug | D01065 |
| PubChem.Substance | 310265165 |
| ChEBI | 46245 |
| ChemSpider | 4445197 |
| Wikipedia | Coenzyme_Q10 |
| HET | U10 |
| DPD | 1357 |