D0488 | crizotinib
L
L01XE16 Crizotinib
[L01XE] Protein kinase inhibitors
[L01X] OTHER ANTINEOPLASTIC AGENTS
[L01] ANTINEOPLASTIC AGENTS
[L] Antineoplastic and immunomodulating agents
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| UNCOUPLING | rat | isolated liver mitochondria | measurements of mitochondrial respiration; RST inhibition assay, RST uncoupling assay; IC 50ratio of glucose/galactose assay | increase | 53 | |||
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
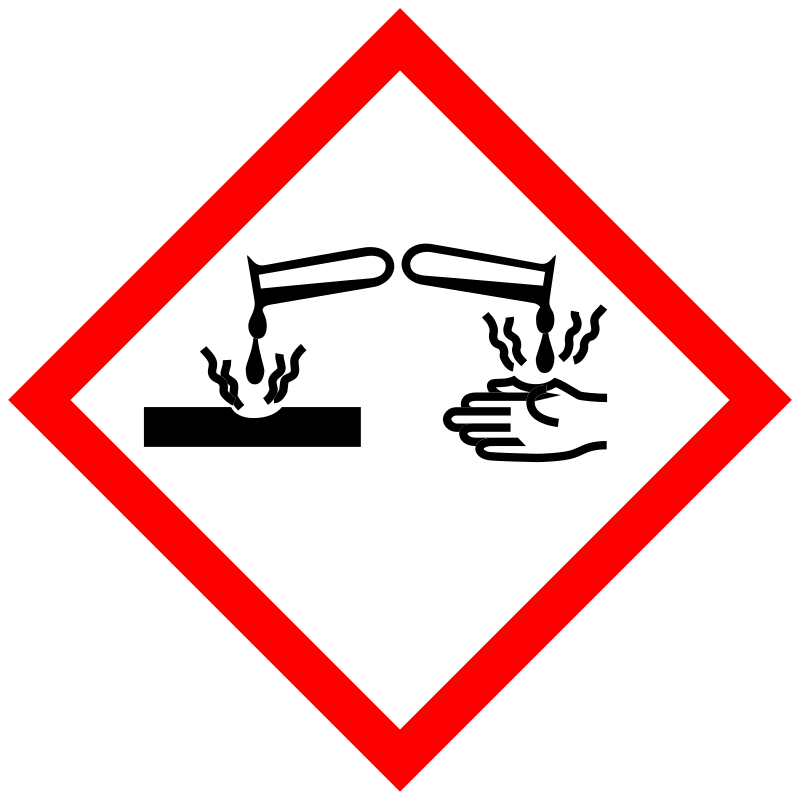    |
Danger |
Aggregated GHS information provided by 71 companies from 4 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H317 (100%): May cause an allergic skin reaction [Warning Sensitization, Skin] H318 (40.85%): Causes serious eye damage [Danger Serious eye damage/eye irritation] H319 (57.75%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H341 (100%): Suspected of causing genetic defects [Warning Germ cell mutagenicity] H400 (59.15%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P261, P264, P272, P273, P280, P281, P302+P352, P305+P351+P338, P308+P313, P310, P321, P333+P313, P337+P313, P363, P391, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
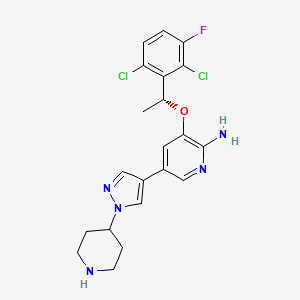
| (R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)pyridin-2-am ine | (R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)pyridin-2-amine | (R)-3-[1-(2,6-Dichloro-3-fluoro-phenyl)-ethoxy]-5-(1-piperidin-4-yl-1H-pyrazol-4-yl)-pyridin-2-ylamine |
| (R)-crizotinib | 2-Pyridinamine, 3-((1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1-(4-piperidinyl)-1H-pyrazol-4-yl)- | 3-(1R)-1-(2,6-Dichloro-3-fluorophenyl)ethoxy-5-1-(4-piperidinyl)-1H-pyrazol-4-yl-2-Pyridinamine |
| 3-(2,6-dichloro-3-fluorobenzyloxy)-5-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)pyridin-2-amine | 3-[(1R)-1-(2,6-DICHLORO-3-FLUOROPHENYL)ETHOXY]-5-[1-(4-PIPERIDINYL)-1H-PYRAZOL-4-YL]PYRIDIN-2-AMINE | 3-[(1R)-1-(2,6-Dichloro-3-fluorophenyl)ethoxy]-5-[1-(4-piperidinyl)-1H-pyrazol-4-yl]-2-pyridinamine |
| 3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-5-[ | 3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-5-[1-(piperidin-4-yl)-1H-pyrazol-4-yl]pyridin-2-amine | 3-[(1r)-1-(2,6-Dichloro-3-Fluorophenyl)ethoxy]-5-(1-Piperidin-4-Yl-1h-Pyrazol-4-Yl)pyridin-2-Amine |
| 3-[(R)-1-(2,6-Dichloro-3-fluoro-phenyl)-ethoxy]-5-(1-piperidin-4-yl-1H-pyrazol-4-yl)-pyridin-2-ylamine; | 3-[1-(2,6-Dichloro-3-fluoro-phenyl)-ethoxy]-5-(1-piperidin-4-yl-1H-pyrazol-4-yl)-pyridin-2-ylamine | 399P525 |
| 53AH36668S | 877399-52-5 | 877399-52-5, 877399-53-6 (acetate) |
| AB0027203 | ABP000131 | AKOS015901233 |
| AKOS015995207 | AMX10152 | ANW-47749 |
| AOB2688 | BB 0261738 | BCPP000116 |
| BDBM50306682 | BRD-K78431006-001-01-1 | BRD-K78431006-001-03-7 |
| C21H22Cl2FN5O | CHEBI:64310 | CHEMBL601719 |
| CTK5F8952 | Crizotinib | Crizotinib (JAN/USAN/INN) |
| Crizotinib (PF-02341066) | Crizotinib (PF-2341066) | Crizotinib [USAN:INN] |
| Crizotinib, >=98% (HPLC) | D09731 | DB08865 |
| DTXSID701009329 | EN002685 | EX-A096 |
| FT-0665224 | GS-6178 | GTPL4903 |
| HY-50878 | J-510370 | KIN0000318 |
| KS-0000075X | KTEIFNKAUNYNJU-GFCCVEGCSA-N | NCGC00250400-01 |
| NCGC00250400-09 | NCGC00250400-12 | NSC-749005 |
| NSC749005 | PB11015 | PF 02341066 |
| PF 2341066 | PF 2341066;(R)-3-[1-(2,6-Dichloro-3-fluoro-phenyl)-ethoxy]-5-(1-piperidin-4-yl-1H-pyrazol-4-yl)-pyridin-2-ylamine | PF-02341066 |
| PF-02341066 (Crizotinib) | PF-2341066 | PF-2341066 (Crizotinib) |
| PF-2341066 - Crizotinib | PF-2341066(Crizotinib) | PF-2341066,Crizotinib |
| PF02341066 | PF2341066 | PubChem19322 |
| Q-3209 | Q5186964 | QCR-32 |
| SC-48062 | SCHEMBL93829 | SW202555-3 |
| SYN1139 | TX-017903 | UNII-53AH36668S |
| VGH | W9013 | Xalkori |
| Xalkori (TN) | ZINC35902489 | crizotinibum |
| DrugBank Name | crizotinib |
| DrugBank | DB08865 |
| CAS Number | 877399-52-5, 877399-53-6 |
| PubChem Compound | 11626560 |
| KEGG Drug | D09731 |
| ChEBI | 64310 |
| PharmGKB | PA165946122 |