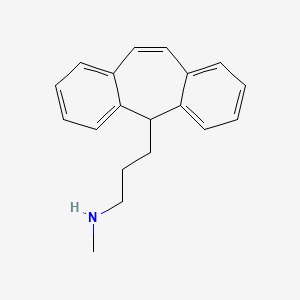
Drug
D0494 | protriptyline
N
N06AA11 Protriptyline
[N06AA] Non-selective monoamine reuptake inhibitors
[N06A] ANTIDEPRESSANTS
[N06] PSYCHOANALEPTICS
[N] Nervous system
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| UNCOUPLING | rat | isolated liver mitochondria | measurements of mitochondrial respiration; RST inhibition assay, RST uncoupling assay; IC 50ratio of glucose/galactose assay | increase | 53 | |||
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| rabbit | LD50 | intravenous | 8200mg/kg (8200mg/kg) | Farmaco, Edizione Pratica. Vol. 25, Pg. 519, 1970. | |
| mouse | LD50 | oral | 269mg/kg (269mg/kg) | Farmaco, Edizione Pratica. Vol. 25, Pg. 519, 1970. | |
| mouse | LD50 | intraperitoneal | 67mg/kg (67mg/kg) | Farmaco, Edizione Pratica. Vol. 25, Pg. 519, 1970. | |
| rat | LD50 | intraperitoneal | 42mg/kg (42mg/kg) | Farmaco, Edizione Pratica. Vol. 25, Pg. 519, 1970. | |
| mouse | LD50 | subcutaneous | 192mg/kg (192mg/kg) | Farmaco, Edizione Pratica. Vol. 25, Pg. 519, 1970. | |
| rat | LD50 | oral | 240mg/kg (240mg/kg) | Farmaco, Edizione Pratica. Vol. 25, Pg. 519, 1970. | |
| mouse | LD50 | intravenous | 30mg/kg (30mg/kg) | behavioral: changes in motor activity (specific assay) | Journal of Medicinal Chemistry. Vol. 17, Pg. 65, 1974. |
| rabbit | LD50 | oral | 310mg/kg (310mg/kg) | Farmaco, Edizione Pratica. Vol. 25, Pg. 519, 1970. | |
| 3-(11H-dibenzo[1,2-a:1',2'-e][7]annulen-11-yl)-N-methylpropan-1-amine | 3-(11H-dibenzo[[?],[?]][7]annulen-11-yl)-N-methyl-propan-1-amine | 3-(5H-Dibenzo[a,d]cyclohepten-5-yl)-N-methyl-1-propanamine |
| 3-(5H-Dibenzo[a,d]cyclohepten-5-yl)-N-methyl-1-propanamine # | 3-(5H-dibenzo[a,d][7]annulen-5-yl)-N-methylpropan-1-amine | 438-60-8 |
| 4NDU154T12 | 5-(3-Methylaminopropyl)-5H-Dibenzo[a,d]cycloheptene | 5-(3-Methylaminopropyl)-5H-dibenzo(a,d)cycloheptene |
| 5H-Dibenzo(a,d)cycloheptene-5-propanamine, N-methyl- | 5H-Dibenzo(a,d)cycloheptene-5-propylamine, N-methyl- | 5H-Dibenzo[a, d]cycloheptene-5-propanamine, N-methyl-, hydrochloride |
| 5H-Dibenzo[a,d]cycloheptene-5-propanamine, N-methyl- | 5H-Dibenzo[a,d]cycloheptene-5-propylamine, N-methyl- | 7-(3-Methylaminopropyl)-1,2:5,6-dibenzocycloheptatriene |
| AB00489964 | AB00489964_10 | AC-15971 |
| ACM438608 | AKOS015962184 | Amimetilina |
| BDBM50176062 | BIDD:PXR0157 | BPBio1_000924 |
| BRD-K42098891-003-03-1 | BRN 2217411 | BSPBio_000840 |
| BWPIARFWQZKAIA-UHFFFAOYSA-N | C07408 | CAS-1225-55-4 |
| CCG-205054 | CHEBI:8597 | CHEMBL668 |
| Concordin | Concordin (Salt/Mix) | D08447 |
| DB00344 | DTXSID0023535 | EINECS 207-119-9 |
| GTPL7285 | HSDB 3391 | L000913 |
| LS-60771 | Lopac-P-8813 | Lopac0_000974 |
| MCULE-6023848462 | MK 240 | MK-240 |
| Maximed | N-3-(5H-Dibenzo(a,d)cyclohepten-5-yl)propyl-N-methylamine | N-Methyl-5H-dibenzo(a,d)cycloheptene-5-propylamine |
| N-Methyl-5H-dibenzo[a,d]cycloheptene-5-propanamine | N-Methyl-5H-dibenzo[a,d]cycloheptene-5-propylamine | NCGC00015851-01 |
| NCGC00015851-02 | NCGC00015851-03 | NCGC00015851-04 |
| NCGC00015851-05 | NCGC00015851-06 | NCGC00015851-07 |
| NCGC00024439-03 | NSC169912 | Normethyl EX4442 |
| PDSP1_001390 | PDSP2_001374 | PROTRYPTYLINE HYDROCHLORIDE |
| Prestwick0_000930 | Prestwick1_000930 | Prestwick2_000930 |
| Prestwick3_000930 | Protriptilina | Protriptilina [INN-Spanish] |
| Protriptyline (INN) | Protriptyline [INN:BAN] | Protriptylinum |
| Protriptylinum [INN-Latin] | Protryptyline | Q408432 |
| SBI-0050947.P002 | SCHEMBL34267 | SPBio_003019 |
| Triptil | Triptil hydrochloride | Triptyl |
| UNII-4NDU154T12 | Vivactil | Vivactil (Salt/Mix) |
| Vivactyl | ZINC1530764 | methyl(3-{tricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8),4,6,9,12,14-heptaen-2-yl}propyl)amine; |
| protriptyline |