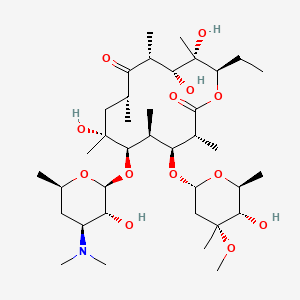
Drug
D1421 | Erythromycin
D
J
S
S01AA17 Erythromycin
[S01AA] Antibiotics
[S01A] ANTIINFECTIVES
[S01] OPHTHALMOLOGICALS
[S] Sensory organs
J01FA01 Erythromycin
[J01FA] Macrolides
[J01F] MACROLIDES, LINCOSAMIDES AND STREPTOGRAMINS
[J01] ANTIBACTERIALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
D10AF52 Erythromycin, combinations
[D10AF] Antiinfectives for treatment of acne
[D10A] ANTI-ACNE PREPARATIONS FOR TOPICAL USE
[D10] ANTI-ACNE PREPARATIONS
[D] Dermatological drugs
D10AF02 Erythromycin
[D10AF] Antiinfectives for treatment of acne
[D10A] ANTI-ACNE PREPARATIONS FOR TOPICAL USE
[D10] ANTI-ACNE PREPARATIONS
[D] Dermatological drugs
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| MEGAMITOCHONDRIA | 200 µg/ml | 22 h | primary culture rat hepatocytes, RL-34,COS-1, IAR-20 | induce | 292 | |||