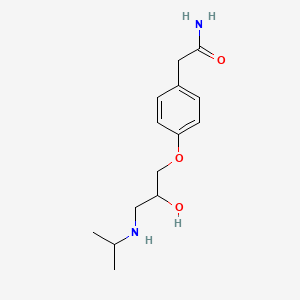
D0011 | Atenolol
C
C07FB03 Atenolol and nifedipine
[C07FB] Beta blocking agents and calcium channel blockers
[C07F] BETA BLOCKING AGENTS, OTHER COMBINATIONS
[C07] BETA BLOCKING AGENTS
[C] Cardiovascular system
C07DB01 Atenolol, thiazides and other diuretics
[C07DB] Beta blocking agents, selective, thiazides and other diuretics
[C07D] BETA BLOCKING AGENTS, THIAZIDES AND OTHER DIURETICS
[C07] BETA BLOCKING AGENTS
[C] Cardiovascular system
C07CB53 Atenolol and other diuretics, combinations
[C07CB] Beta blocking agents, selective, and other diuretics
[C07C] BETA BLOCKING AGENTS AND OTHER DIURETICS
[C07] BETA BLOCKING AGENTS
[C] Cardiovascular system
C07CB03 Atenolol and other diuretics
[C07CB] Beta blocking agents, selective, and other diuretics
[C07C] BETA BLOCKING AGENTS AND OTHER DIURETICS
[C07] BETA BLOCKING AGENTS
[C] Cardiovascular system
C07BB03 Atenolol and thiazides
[C07BB] Beta blocking agents, selective, and thiazides
[C07B] BETA BLOCKING AGENTS AND THIAZIDES
[C07] BETA BLOCKING AGENTS
[C] Cardiovascular system
C07AB03 Atenolol
[C07AB] Beta blocking agents, selective
[C07A] BETA BLOCKING AGENTS
[C07] BETA BLOCKING AGENTS
[C] Cardiovascular system
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| GLUCOSE GALACTOSE IC50 RATIO | 300.0 ± 0, 300.0 ± 0, 1, 300.0 ± 0, 300.0 ± 0, 1 | 4hr | H9c2 cells | high-glucose–galactose cell viability assay with mitochondrial membrane potential (JC-1) and ATP-depletion assays (CellTiter-Glo reagent ). | Negative | glucose/galactose IC50 ratio (JC-1 IC50 in glucose, JC-1 IC50 in galactose, JC-1 glu/gla, ATP IC50 in glucose, ATP IC50 in galactose, ATP glu/gla )(μM) | 50 | |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Warning |
Aggregated GHS information provided by 87 companies from 22 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. Reported as not meeting GHS hazard criteria by 3 of 87 companies. For more detailed information, please visit ECHA C&L website Of the 20 notification(s) provided by 84 of 87 companies with hazard statement code(s): H302 (82.14%): Harmful if swallowed [Warning Acute toxicity, oral] H319 (69.05%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H351 (67.86%): Suspected of causing cancer [Warning Carcinogenicity] H362 (77.38%): May cause harm to breast-fed children [Reproductive toxicity, effects on or via lactation] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P260, P263, P264, P270, P280, P281, P301+P312, P305+P351+P338, P308+P313, P330, P337+P313, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | subcutaneous | > 400mg/kg (400mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 8, Pg. 4579, 1980. | |
| women | TDLo | oral | 16mg/kg (16mg/kg) | lungs, thorax, or respiration: respiratory obstruction | Annals of Emergency Medicine. Vol. 14, Pg. 161, 1985. |
| mouse | LD50 | intraperitoneal | 134mg/kg (134mg/kg) | Toksikologicheskii Vestnik. Vol. (4), Pg. 33, 1995. | |
| rat | LD50 | intravenous | 84mg/kg (84mg/kg) | PCT Vol. #91-10428, | |
| women | TDLo | oral | 1080mg/kg/78W (1080mg/kg) | Journal of Rheumatology. Vol. 13, Pg. 446, 1986. | |
| rat | LD50 | intravenous | 77mg/kg (77mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 8, Pg. 4579, 1980. | |
| rat | LD50 | oral | > 2gm/kg (2000mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 8, Pg. 4579, 1980. | |
| mouse | LD50 | oral | 2gm/kg (2000mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 8, Pg. 4579, 1980. | |
| man | TDLo | oral | 10204ug/kg (10.204mg/kg) | Anesthesiology. Vol. 83, Pg. 204, 1995. | |
| rat | LD50 | subcutaneous | > 600mg/kg (600mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 8, Pg. 4579, 1980. | |
| women | TDLo | oral | 42mg/kg/3W-I (42mg/kg) | British Medical Journal. Vol. 294, Pg. 1324, 1987. | |
| mouse | LD50 | intravenous | 57mg/kg (57mg/kg) | peripheral nerve and sensation: flaccid paralysis without anesthesia (usually neuromuscular blockage) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 8, Pg. 4579, 1980. |
| rabbit | LD50 | intravenous | 50mg/kg (50mg/kg) | Drugs. International Journal of Current Therapeutics and Applied Pharmacology Reviews. Vol. 17, Pg. 425, 1979. | |
| rat | LD50 | intravenous | 97mg/kg (97mg/kg) | PCT Vol. #91-10428, | |
| man | TDLo | oral | 129mg/kg/26W- (129mg/kg) | Annales de Medecine Interne. Vol. 148, Pg. 505, 1997. | |
| man | TDLo | oral | 86mg/kg/60D-I (86mg/kg) | blood: "changes in serum composition (e.g., tp, bilirubin, cholesterol)" | American Journal of Medicine. Vol. 85, Pg. 586, 1988. |
| man | TDLo | oral | 49mg/kg/30D-I (49mg/kg) | behavioral: excitement | Journal of Clinical Pyschopharmacology. Vol. 6, Pg. 390, 1986. |
| ( inverted question mark)-Atenolol | (+)-4-[2-Hydroxy-3-[(1-methylethyl)amino]propoxy]benzeneacetamide | (+-)-4-(2-Hydroxy-3-isopropylaminopropoxy)phenylacetamide |
| (+-)-Atenolol | (+/-)-4-(2-Hydroxy-3-[(1-methylethyl)amino]propoxy)benzeneacetamide | (+/-)-4-[2-Hydroxy-3-[(1-methylethyl)amino]propoxy]benzeneacetamide |
| (+/-)-Atenolol | (1)-2-(4-(2-Hydroxy-3-(isopropylamino)propoxy)phenyl)acetamide | (?)-Atenolol |
| (A+/-)-Atenolol | (RS)-4-[2-Hydroxy-3-[(1-methylethyl)amino]propoxy]benzeneacetamide | (RS)-Atenolol |
| (r,s)-atenolol | (y)-Atenolol | 1-p-Carbamoylmethylphenoxy-3-isopropylamino-2-propanol |
| 122A687 | 2-(4-(2-Hydroxy-3-isopropylaminopropoxy)phenyl)acetamid | 2-(4-(2-hydroxy-3-(isopropylamino)propoxy) |
| 2-(4-(2-hydroxy-3-(isopropylamino)propoxy)phenyl)acetamide | 2-(4-[2-Hydroxy-3-(isopropylamino)propoxy]phenyl)acetamide | 2-(4-{2-hydroxy-3-[(methylethyl)amino]propoxy}phenyl)acetamide |
| 2-(4-{2-hydroxy-3-[(propan-2-yl)amino]propoxy}phenyl)acetamide | 2-(4-{[(2S)-2-hydroxy-3-(propan-2-ylamino)propyl]oxy}phenyl)acetamide | 2-(p-(2-Hydroxy-3-(isopropylamino)propoxy)phenyl)acetamide |
| 2-[4-(2-Hydroxy-3-isopropylaminopropoxy)phenyl]acetamide | 2-[4-({(2R)-2-hydroxy-3-[(1-methylethyl)amino]propyl}oxy)phenyl]acetamide | 2-[4-({2-hydroxy-3-[(1-methylethyl)amino]propyl}oxy)phenyl]acetamide |
| 2-[4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl]acetamide | 2-{4-[2-Hydroxy-3-(isopropylamino)propoxy]-phenyl}acetamide | 2-{4-[2-hydroxy-3-(isopropylamino)propoxy]phenyl}acetamide |
| 2-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl}acetamide | 29122-68-7 | 4-(2-Hydroxy-3-((1-methylethyl)amino)propoxy)benzeneacetamide |
| 4-[2'-Hydroxy-3'-(isopropylamino)propoxy]phenylacetamide | 60966-51-0 | A 7655 |
| AB00052208-13 | AB00052208-15 | AB00052208_16 |
| AB0011597 | AC-8245 | AKOS005111050 |
| ANW-45215 | API0001553 | ARONIS23884 |
| AX8005715 | Acetamide, 2-(p-(2-hydroxy-3-(isopropylamino)propoxy)phenyl)- | Acetamide,2-(p-(2-hydroxy-3-(isopropylamino)propoxy)phenyl) |
| Aircrit | Alinor | Altol |
| Anselol | Antipressan | Apo-Atenolol |
| Artrenolol | Atcard | Atcardil |
| Atecard | Atehexal | Atenblock |
| Atendol | Atenet | Ateni |
| Atenil | Atenol | Atenol 1A pharma |
| Atenol AL | Atenol Atid | Atenol Cophar |
| Atenol Fecofar | Atenol GNR | Atenol Gador |
| Atenol Genericon | Atenol Heumann | Atenol MSD |
| Atenol NM Pharma | Atenol Nordic | Atenol PB |
| Atenol Quesada | Atenol Stada | Atenol Tika |
| Atenol Trom | Atenol acis | Atenol ct |
| Atenol von ct | Atenol-Mepha | Atenol-Wolff |
| Atenol-ratiopharm | Atenolin | Atenolol (JAN/USP) |
| Atenolol (JP17/USP/INN) | Atenolol 1.0 mg/ml in Acetonitrile | Atenolol 100 microg/mL in Methanol |
| Atenolol Bp | Atenolol [USAN:BAN:INN:JAN] | Atenolol [USAN:INN:BAN:JAN] |
| Atenolol [USAN:USP:INN:BAN:JAN] | Atenolol solution, 1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material | Atenolol, 98% |
| Atenolol, >=98% (TLC), powder | Atenolol, European Pharmacopoeia (EP) Reference Standard | Atenolol, Pharmaceutical Secondary Standard |
| Atenolol, United States Pharmacopeia (USP) Reference Standard | Atenolol, analytical reference material | Atenolol,(S) |
| Atenololum | Atenololum [INN-Latin] | Atenomel |
| Atereal | Aterol | BBC/163 |
| BBL009276 | BB_SC-01519 | BCP12899 |
| BDBM25753 | BENZENEACETAMIDE, 4-(2-HYDROXY-3-((1-METHYLETHYL)AMINO)PROPOXY)-, (+-)- | BG0049 |
| BIM-0050109.0001 | BRD-A20239487-001-02-5 | BRD-A20239487-001-15-7 |
| BRN 2739235 | BSPBio_002915 | Benzeneacetamide, 4-(2-hydroxy-3-((1-methylethyl)amino)propoxy)- |
| Benzeneacetamide, 4-[2-hydroxy-3-[(1-methylethyl)amino]propoxy]- | Betablok | Betacard |
| Betasyn | Betatop Ge | Blocotenol |
| Blokium | CA0145 | CAS-29122-68-7 |
| CCG-39010 | CCRIS 4196 | CHEBI:2904 |
| CHEMBL24 | CPD000036768 | CS-2927 |
| CTK3J2163 | Cardaxen | Cardiopress |
| Certified Reference Material | Corotenol | Cuxanorm |
| D00235 | DB-072177 | DB-079552 |
| DB00335 | DSSTox_CID_2628 | DSSTox_GSID_22628 |
| DSSTox_RID_76663 | DTXSID2022628 | DivK1c_000057 |
| Duraatenolol | EINECS 249-451-7 | EINECS 262-544-7 |
| EN300-119532 | EU-0100121 | Evitocor |
| FT-0622499 | FT-0693045 | Farnormin |
| Felo-Bits | GEO-03413 | GTPL548 |
| HMS1569L13 | HMS1921H09 | HMS2090I19 |
| HMS2092D19 | HMS2233E06 | HMS3259K08 |
| HMS3260I04 | HMS3266K13 | HMS3369B14 |
| HMS3369D20 | HMS3369P20 | HMS3411G21 |
| HMS3675G21 | HMS500C19 | HSDB 6526 |
| HY-17498 | Hipres | Hypoten |
| ICI 66,082 | ICI 66082 | ICI-66082 |
| IDI1_000057 | Ibinolo | InChI=1/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18) |
| Internolol | J10009 | Jenatenol |
| Juvental | KBio1_000057 | KBio2_001844 |
| KBio2_004412 | KBio2_006980 | KBio3_002415 |
| KBioGR_000790 | KBioSS_001844 | KS-00000XIG |
| KS-000046IQ | KS-5341 | KSC492C6H |
| L000116 | LP00121 | LS-28557 |
| LS-9707 | Lo-ten | Lopac0_000121 |
| Loten | Lotenal | MCULE-9148694071 |
| METKIMKYRPQLGS-UHFFFAOYSA- | METKIMKYRPQLGS-UHFFFAOYSA-N | MFCD00057645 |
| MLS000069622 | MLS001066372 | MLS001074163 |
| MLS001304038 | MRF-0000571 | Myocord |
| NC00548 | NCGC00015007-06 | NCGC00015007-07 |
| NCGC00015007-08 | NCGC00015007-09 | NCGC00015007-10 |
| NCGC00015007-11 | NCGC00015007-13 | NCGC00024566-03 |
| NCGC00024566-04 | NCGC00024566-05 | NCGC00024566-06 |
| NCGC00024566-07 | NCGC00255122-01 | NCGC00260806-01 |
| NINDS_000057 | NSC-757832 | NSC757832 |
| Neatenol | Normalol | Normiten |
| Noten | Opera_ID_1283 | Oprea1_448775 |
| Oraday | Ormidol | Panapres |
| Pharmakon1600-01501127 | Plenacor | Premorine |
| Prenolol | Prenormine | Prinorm |
| Q-200656 | Q411325 | RTR-012731 |
| SAM002564193 | SBB056998 | SBI-0050109.P003 |
| SC-19545 | SCHEMBL4362 | SMR000036768 |
| SPBio_001482 | SPECTRUM1501127 | SR-01000000159 |
| SR-01000000159-2 | SR-01000000159-4 | SR-01000000159-5 |
| SR-01000000159-8 | ST024754 | ST2403914 |
| STK528649 | Scheinpharm Atenol | Seles beta |
| Selobloc | Serten | Servitenol |
| Spectrum2_001411 | Spectrum3_001448 | Spectrum4_000435 |
| Spectrum5_001509 | Spectrum_001364 | Stermin |
| TR-012731 | Tenidon | Teno-basan |
| Tenobloc | Tenoblock | Tenolol |
| Tenoprin | Tenoretic (Salt/Mix) | Tenormin |
| Tenormin (TN) | Tenormine | Tenormine [French] |
| Tensimin | Tensotin | Tox21_302426 |
| Tox21_500121 | Tredol | Unibloc |
| Uniloc | Vascoten | Vericordin |
| Wesipin | Xaten | Z-0828 |
| Z1541638518 | ZX-AS004380 | atenolol |
| benzeneacetamide, 4-[2'-hydroxy-3;-[(1-methylethyl)amino]propoxy]- | dl-Atenolol | duratenol |
| phenyl)acetamide |