Drug
D0027 | Cinnarizine
N
N07CA52 Cinnarizine, combinations
[N07CA] Antivertigo preparations
[N07C] ANTIVERTIGO PREPARATIONS
[N07] OTHER NERVOUS SYSTEM DRUGS
[N] Nervous system
N07CA02 Cinnarizine
[N07CA] Antivertigo preparations
[N07C] ANTIVERTIGO PREPARATIONS
[N07] OTHER NERVOUS SYSTEM DRUGS
[N] Nervous system
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| ELECTRON TRANSPORT CHAIN | 5–10 μM | NADH–Q-1 | decrease | IC50 | 121 | |||
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| NADH:ubiquinone reductase | 5–10 μM | NADH–Q-1 | inhibitor | IC50 | 121 | |||
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| women | TDLo | oral | 22500mg/kg/22 (22500mg/kg) | Annals of Pharmacotherpy. Vol. 26, Pg. 928, 1992. | |
| rat | LD50 | intraperitoneal | 1050mg/kg (1050mg/kg) | Medicamentos de Actualidad. Vol. 21, Pg. 443, 1985. | |
| mouse | LD50 | subcutaneous | > 2gm/kg (2000mg/kg) | Drugs in Japan Vol. -, Pg. 515, 1990. | |
| rat | LD50 | oral | > 6500mg/kg (6500mg/kg) | Kiso to Rinsho. Clinical Report. Vol. 16, Pg. 1735, 1982. | |
| mouse | LD50 | intravenous | 22mg/kg (22mg/kg) | Kiso to Rinsho. Clinical Report. Vol. 16, Pg. 1735, 1982. | |
| rat | LD50 | oral | 400mg/kg (400mg/kg) | National Technical Information Service. Vol. AD-A082-824, | |
| dog | LD50 | intraperitoneal | > 500mg/kg (500mg/kg) | Drugs in Japan Vol. -, Pg. 515, 1990. | |
| mouse | LD50 | oral | > 4500mg/kg (4500mg/kg) | Kiso to Rinsho. Clinical Report. Vol. 16, Pg. 1735, 1982. | |
| rat | LD | skin | > 5600mg/kg (5600mg/kg) | Journal of the American College of Toxicology. Vol. 12, Pg. 369, 1993. | |
| mouse | LD50 | intraperitoneal | 730mg/kg (730mg/kg) | Medicamentos de Actualidad. Vol. 21, Pg. 443, 1985. | |
| mouse | LD50 | oral | 606mg/kg (606mg/kg) | National Technical Information Service. Vol. AD-A082-824, | |
| rat | LD50 | subcutaneous | > 2gm/kg (2000mg/kg) | Drugs in Japan Vol. -, Pg. 515, 1990. | |
| dog | LD50 | oral | > 500mg/kg (500mg/kg) | Drugs in Japan Vol. -, Pg. 515, 1990. | |
| dog | LD50 | subcutaneous | > 500mg/kg (500mg/kg) | Drugs in Japan Vol. -, Pg. 515, 1990. | |
| women | TDLo | oral | 252mg/kg/12W- (252mg/kg) | skin and appendages (skin): "dermatitis, allergic: after systemic exposure" | British Journal of Dermatology. Vol. 112, Pg. 607, 1985. |
| rat | LD50 | intravenous | 24mg/kg (24mg/kg) | Kiso to Rinsho. Clinical Report. Vol. 16, Pg. 1735, 1982. | |
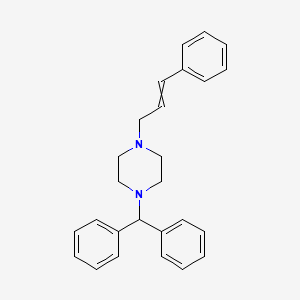
| 1-(Diphenylmethyl)-4-(3-phenyl-2-propen-1-yl)piperazine | 1-(diphenylmethyl)-4-(3-phenyl-2-propenyl) piperazine | 1-(diphenylmethyl)-4-(3-phenylprop-2-enyl)piperazine |
| 1-Cinnamyl-4-diphenylmethylpiperazine | C3459 | CTK4D2521 |
| DB-047662 | DB00568 | FT-0623849 |
| FT-0665054 | HMS3372L16 | KS-000048IA |
| MCULE-5586055809 | Oprea1_696883 | Piperazine,1-(diphenylmethyl)-4-[(2E)-3-phenyl-2-propen-1-yl]- |
| Prestwick0_000278 | Prestwick1_000278 | Q27224950 |
| SPBio_002375 |
| DrugBank Name | Cinnarizine |
| DrugBank | DB00568 |
| CAS Number | 16699-20-0, 298-57-7, 750512-44-8, 98-57-7 |
| PubChem Compound | 2761 |
| KEGG Drug | D01295 |
| PubChem.Substance | 46506769 |
| ChEBI | 31403 |
| PharmGKB | PA164749342 |
| ChemSpider | 1264793 |
| BindingDB | 50017657.0 |
| TTD | DAP000325 |
| Wikipedia | Cinnarizine |
1. Chan et al. (2005)