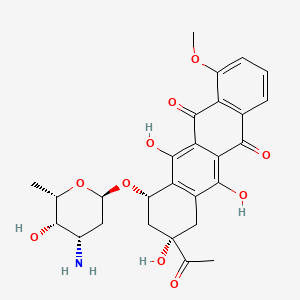
D0036 | Daunorubicin
L
L01XY01 Cytarabine and daunorubicin
[L01XY] Combinations of antineoplastic agents
[L01X] OTHER ANTINEOPLASTIC AGENTS
[L01] ANTINEOPLASTIC AGENTS
[L] Antineoplastic and immunomodulating agents
L01DB02 Daunorubicin
[L01DB] Anthracyclines and related substances
[L01D] CYTOTOXIC ANTIBIOTICS AND RELATED SUBSTANCES
[L01] ANTINEOPLASTIC AGENTS
[L] Antineoplastic and immunomodulating agents
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| MEMBRANE POTENTIAL | 30 mins | mouse | liver mitochondria | Rh123 fluorescence (excitation 485 nm, emission 535 nm) are recorded using a fluorescence multi-well plate reader (mCICCP (20 µM) treatments was considered as the 100% baseline for ΔΨm loss) | Negative | EC20 | 36 | |
| RESPIRATION | 12.8 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | decrease | EC20 | 36 |
| RESPIRATION | 10.9 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | decrease | EC20 | 36 |
| SWELLING | < 6.25 µM | 30 mins | mouse | liver mitochondria | swelling assay: Absorbance at 545 nm using a fluorescence multi-well plate reader (CaCl2 (50 µM) was considered as the 100% baseline for the swelling ) | increase | EC20 | 36 |
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| NADH:ubiquinone reductase | 12.8 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | inhibit | EC20 | 36 |
| Succinate dehydrogenase | 10.9 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | inhibit | EC20 | 36 |
| Cytochrome c | 47.2 µM | 30 mins | mouse | liver mitochondria | Cytochrome c release was evaluated using ELISA kit ( 20 µg/ml Alamethicin was used as 100% baseline) | release | EC20 | 36 |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Danger |
Aggregated GHS information provided by 52 companies from 1 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H301 (100%): Toxic if swallowed [Danger Acute toxicity, oral] H341 (100%): Suspected of causing genetic defects [Warning Germ cell mutagenicity] H351 (100%): Suspected of causing cancer [Warning Carcinogenicity] H361 (100%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P264, P270, P281, P301+P310, P308+P313, P321, P330, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 205mg/kg (205mg/kg) | Yakkyoku. Pharmacy. Vol. 25, Pg. 573, 1974. | |
| rat | LD50 | oral | 336mg/kg (336mg/kg) | Yakkyoku. Pharmacy. Vol. 25, Pg. 573, 1974. | |
| dog | LD50 | intravenous | 4mg/kg (4mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 17, Pg. 948, 1967. | |
| guinea pig | LD50 | intravenous | 6mg/kg (6mg/kg) | behavioral: somnolence (general depressed activity) | Arzneimittel-Forschung. Drug Research. Vol. 17, Pg. 948, 1967. |
| child | LDLo | oral | 10mg/kg/30D-I (10mg/kg) | cardiac: cardiomyopathy including infarction | British Journal of Clinical Practice. Vol. 44, Pg. 633, 1990. |
| mouse | LD50 | unreported | 24900ug/kg (24.9mg/kg) | Biochemical Pharmacology. Vol. 38, Pg. 167, 1989. | |
| hamster | LDLo | intravenous | 50mg/kg (50mg/kg) | Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 15, Pg. 1614, 1973. | |
| mouse | LD50 | intravenous | 8600ug/kg (8.6mg/kg) | Cancer Research. Vol. 49, Pg. 4098, 1989. | |
| rat | LD50 | intravenous | 13mg/kg (13mg/kg) | National Cancer Institute Report. Vol. -, Pg. 304, 1967. | |
| rat | LD50 | subcutaneous | 33200ug/kg (33.2mg/kg) | Yakkyoku. Pharmacy. Vol. 25, Pg. 573, 1974. | |
| human | LDLo | oral | 6mg/kg (6mg/kg) | cardiac: other changes | "Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 521, 1969. |
| rat | LD50 | intraperitoneal | 20mg/kg (20mg/kg) | Advances in Teratology. Vol. 3, Pg. 181, 1968. | |
| mouse | LD50 | intraperitoneal | 2500ug/kg (2.5mg/kg) | gastrointestinal: other changes | National Cancer Institute Report. Vol. -, Pg. 304, 1967. |
| rabbit | LD50 | intravenous | 5mg/kg (5mg/kg) | behavioral: somnolence (general depressed activity) | Arzneimittel-Forschung. Drug Research. Vol. 17, Pg. 948, 1967. |
| (+)-Daunomycin | (1S,3S)-3-acetyl-3,5,12-trihydroxy-10-(methyloxy)-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside | (1S,3S)-3-acetyl-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-a-L-lyxo-hexopyranoside |
| (1S,3S)-3-acetyl-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside | (7S,9R)-9-Acetyl-7-[(2S,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-oxan-2-yl]oxy-6,9,11-trihydroxy-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione | (7S,9S)-7-[(2R,4S,5S,6S)-4-azanyl-6-methyl-5-oxidanyl-oxan-2-yl]oxy-9-ethanoyl-4-methoxy-6,9,11-tris(oxidanyl)-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride |
| (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-6,9,11-trihydroxy-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione | (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-6,9,11-trihydroxy-4-methoxy-8,10-dihydro-7H-tetracene-5,12-quinone;hydrochloride | (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride |
| (7S,9S)-9-acetyl-7-[[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-2-oxanyl]oxy]-6,9,11-trihydroxy-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride | (8S,10S)-8-acetyl-10-(((2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-6,8,11-trihydroxy-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione | (8S,10S)-8-acetyl-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy}-6,8,11-trihydroxy-1-methoxy-5,7,8,9,10,12-hexahydrotetracene-5,12-dione |
| (8S-cis)-8-Acetyl-10-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyrannosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-napthacenedione | (8S-cis)-8-Acetyl-10-[(3-amino-2,3,6-trideoxy-.alpha.-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro--6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione | 1407-15-4 |
| 15159-EP2270008A1 | 15159-EP2272827A1 | 15159-EP2277565A2 |
| 15159-EP2277566A2 | 15159-EP2277567A1 | 15159-EP2277568A2 |
| 15159-EP2277569A2 | 15159-EP2277570A2 | 15159-EP2289892A1 |
| 15159-EP2292280A1 | 15159-EP2292617A1 | 15159-EP2295416A2 |
| 15159-EP2295426A1 | 15159-EP2295427A1 | 15159-EP2298748A2 |
| 15159-EP2298778A1 | 15159-EP2301928A1 | 15159-EP2305642A2 |
| 15159-EP2305679A1 | 15159-EP2308833A2 | 15159-EP2308861A1 |
| 15159-EP2311808A1 | 15159-EP2311829A1 | 15159-EP2311842A2 |
| 15159-EP2316832A1 | 15159-EP2316833A1 | 16803-EP2272832A1 |
| 16803-EP2277565A2 | 16803-EP2277566A2 | 16803-EP2277567A1 |
| 16803-EP2277568A2 | 16803-EP2277569A2 | 16803-EP2277570A2 |
| 16803-EP2280012A2 | 16803-EP2281815A1 | 16803-EP2286812A1 |
| 16803-EP2292280A1 | 16803-EP2292615A1 | 16803-EP2298768A1 |
| 16803-EP2301928A1 | 16803-EP2301933A1 | 16803-EP2305640A2 |
| 16803-EP2305671A1 | 16803-EP2311825A1 | 16803-EP2311827A1 |
| 16803-EP2311840A1 | 16803-EP2316937A1 | 20830-81-3 |
| 30D813 | 5,12-Naphthacenedione, 8-acetyl-10-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-, (8S,10S)- | 5,12-Naphthacenedione, 8-acetyl-10-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-, (8S-cis)- |
| AB00514669 | AB00514669-09 | AB01644616_09 |
| AB01644616_10 | AI3-52942 | API0005415 |
| AX8123492 | Acetyladriamycin | Anthracyline |
| BDBM32017 | BDBM50368352 | BPBio1_000389 |
| BRD-K43389675-001-01-3 | BRD-K43389675-003-02-7 | BRD-K43389675-003-03-5 |
| BRD-K43389675-003-20-9 | BRN 1445583 | BSPBio_000353 |
| C01907 | C27H29NO10 | CAS-20830-81-3 |
| CCG-212559 | CCRIS 914 | CHEBI:41977 |
| CHEMBL178 | CS-2004 | Cerubidin |
| Cerubidine | D07776 | DB00694 |
| DSSTox_CID_2883 | DSSTox_GSID_22883 | DSSTox_RID_76773 |
| DTXSID7022883 | Daunamycin | Daunarubicinum |
| Dauno-Rubidomycine | DaunoXome | DaunoXome (TN) |
| Daunoblastin | Daunoblastine | Daunomycin |
| Daunorrubicina | Daunorubicin (INN) | Daunorubicin (liposomal) |
| Daunorubicin [INN:BAN] | Daunorubicin(Daunomycin) | Daunorubicina |
| Daunorubicine | Daunorubicinum | Daunorubicinum [INN-Latin] |
| EINECS 244-069-7 | FI 6339 | FI-6339 |
| FI6339 | FT-0624457 | GR-318 |
| GTPL7063 | HMS2089H04 | HMS2091K06 |
| HSDB 5095 | HY-13062A | LMPK13050002 |
| LS-187381 | LS-997 | Leukaemomycin C |
| MLS000069508 | NCGC00024246-05 | NCGC00024246-06 |
| NCGC00024246-07 | NCGC00024246-09 | NCGC00024246-10 |
| NCGC00024246-15 | NCGC00025173-01 | NCI-C04693 |
| NDC 0082-4155 | NDC-0082-4155 | NSC 83142 |
| NSC-756717 | NSC-82151 | NSC756717 |
| NSC82151 | Pharmakon1600-01500223 | Prestwick3_000487 |
| Q411659 | RCRA waste no. U059 | RP 13057 |
| RP-13057 | Rubidomycin | Rubomycin |
| Rubomycin C | SBI-0206677.P002 | SC-18793 |
| SCHEMBL3041 | SMR000058559 | SR-01000000033 |
| SR-01000000033-4 | SR-05000001600 | SR-05000001600-1 |
| SR-05000001600-2 | STQGQHZAVUOBTE-VGBVRHCVSA-N | Tocris-1467 |
| Tox21_110896 | Tox21_110896_1 | UNII-ZS7284E0ZP |
| VS-103 | ZINC3917708 | ZS7284E0ZP |
| cid_62770 | daunorubicin |
| DrugBank Name | Daunorubicin |
| DrugBank | DB00694 |
| CAS Number | 1407-15-4, 20830-81-3 |
| PubChem Compound | 30323 |
| KEGG Compound ID | C01907 |
| KEGG Drug | D07776 |
| PubChem.Substance | 46508433 |
| ChEBI | 41977 |
| PharmGKB | PA449212 |
| ChemSpider | 28163 |
| BindingDB | 32017.0 |
| TTD | DNC000517 |
| Wikipedia | Daunorubicin |
| HET | DM1 |
| DPD | 68 |