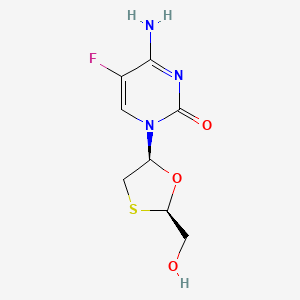
D0046 | Emtricitabine
J
J05AR22 Emtricitabine, tenofovir alafenamide, darunavir and cobicistat
[J05AR] Antivirals for treatment of HIV infections, combinations
[J05A] DIRECT ACTING ANTIVIRALS
[J05] ANTIVIRALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
J05AR20 Emtricitabine, tenofovir alafenamide and bictegravir
[J05AR] Antivirals for treatment of HIV infections, combinations
[J05A] DIRECT ACTING ANTIVIRALS
[J05] ANTIVIRALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
J05AR19 Emtricitabine, tenofovir alafenamide and rilpivirine
[J05AR] Antivirals for treatment of HIV infections, combinations
[J05A] DIRECT ACTING ANTIVIRALS
[J05] ANTIVIRALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
J05AR18 Emtricitabine, tenofovir alafenamide, elvitegravir and cobicistat
[J05AR] Antivirals for treatment of HIV infections, combinations
[J05A] DIRECT ACTING ANTIVIRALS
[J05] ANTIVIRALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
J05AR17 Emtricitabine and tenofovir alafenamide
[J05AR] Antivirals for treatment of HIV infections, combinations
[J05A] DIRECT ACTING ANTIVIRALS
[J05] ANTIVIRALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
J05AR09 Emtricitabine, tenofovir disoproxil, elvitegravir and cobicistat
[J05AR] Antivirals for treatment of HIV infections, combinations
[J05A] DIRECT ACTING ANTIVIRALS
[J05] ANTIVIRALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
J05AR08 Emtricitabine, tenofovir disoproxil and rilpivirine
[J05AR] Antivirals for treatment of HIV infections, combinations
[J05A] DIRECT ACTING ANTIVIRALS
[J05] ANTIVIRALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
J05AR06 Emtricitabine, tenofovir disoproxil and efavirenz
[J05AR] Antivirals for treatment of HIV infections, combinations
[J05A] DIRECT ACTING ANTIVIRALS
[J05] ANTIVIRALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
J05AR03 Tenofovir disoproxil and emtricitabine
[J05AR] Antivirals for treatment of HIV infections, combinations
[J05A] DIRECT ACTING ANTIVIRALS
[J05] ANTIVIRALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
J05AF09 Emtricitabine
[J05AF] Nucleoside and nucleotide reverse transcriptase inhibitors
[J05A] DIRECT ACTING ANTIVIRALS
[J05] ANTIVIRALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| MITOCHONDRIAL DNA METABOLIC PROCESS | 194 | |||||||
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| DNA polymerase gamma | 194 | |||||||
| ((-))-FTC | (+)-2'-Deoxy-3'-thia-5-fluorocytidine | (-)-(2R,5S)-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine |
| (-)-.beta.-L-FTC | (-)-2'-Deoxy-5-fluoro-3'-thiacytidine | (-)-BETA-2',3'-DIDEOXY-5-FLUORO-3'-THIACYTIDINE |
| (-)-FTC | (-)-beta-2',3'-dideoxy-5-fluoro-3'-thiacytidine | (-)-cis-4-amino-5-fluoro-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one |
| (2R-cis)-4-Amino-5-fluoro-1-(2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-2(1H)-pyrimidinone | (2S-cis)-4-Amino-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-2(1H)-pyrimidinone | .beta.-L-(-)-(2R,5S)-5-Fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine |
| 1-(2-(Hydroxymethyl)oxathiolan-5-yl)-5-fluorocytosine | 143491-54-7 | 143491-57-0 |
| 145213-48-5 | 2',3',5-FTC | 2',3'-Dideoxy-3-thia-5-fluorocytidine |
| 2',3'-Dideoxy-5-fluoro-3'-thiacytidine | 2'-Deoxy-5-fluoro-3'-oxa-4'-thiocytidine | 2'-Deoxy-5-fluoro-3'-thiacytidine |
| 2(1H)-Pyrimidinone, 4-amino-5-fluoro-1-((2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-, rel- | 2(1H)-Pyrimidinone, 4-amino-5-fluoro-1-(2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-, (2R-cis)- | 2(1H)-Pyrimidinone, 4-amino-5-fluoro-1-(2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-, cis- |
| 2(1H)-Pyrimidinone, 4-amino-5-fluoro-1-(2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-, cis-(+-)- | 2(1H)-Pyrimidinone, 4-amino-5-fluoro-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-, rel- | 2(1H)-Pyrimidinone,4-amino-5-fluoro-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]- |
| 2-FTC | 24229-EP2298783A1 | 24229-EP2314590A1 |
| 3'-Thia-2'.3'-dideoxy-5-fluorocytidine | 4-Amino-5-fluoro-1-((2R,5S)-2-hydroxymethyl-[1,3]oxathiolan-5-yl)-1H-pyrimidin-2-one | 4-Amino-5-fluoro-1-((2R,5S)-rel-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)pyrimidin-2(1H)-one |
| 4-Amino-5-fluoro-1-((2S,5R)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)pyrimidin-2(1H)-one | 4-Amino-5-fluoro-1-[(2R,5S)-2-hydroxymethyl)-1,3-oxathiolan-5-yl]-2-(1H-pyrimidinone | 4-Amino-5-fluoro-1-[(2S,5R)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-2(1H)-pyrimidinone |
| 4-amino-5-fluoro-1-((2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)pyrimidin-2(1H)-one | 4-amino-5-fluoro-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-1,2-dihydropyrimidin-2-one | 4-amino-5-fluoro-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2(1H)-one |
| 4-amino-5-fluoro-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one | 491E570 | 5-FLUORO-(-)-L-2',3'-DIDEOXY-3'-THIACYTIDINE |
| 5-Fluoro-1-((2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)cytosine | 5-Fluoro-1-(2-(hydroxymethyl)-1,3-oxathiolan-5-yl)cytosine | 5-fluoro-1-[(2R,5S)-2-(hydroxymethyl)[1,3]oxathiolan-5-yl]cytosine |
| 524W91 | AB01275429-01 | ABP001074 |
| AKOS015853098 | AKOS015894950 | AM84393 |
| ANW-58684 | AS-14099 | BDBM50107843 |
| BW 1592 | BW 524W91 | BW-524W91 |
| BW1592 | BW524W91 | C12599 |
| C8H10FN3O3S | CAS-143491-57-0 | CCG-220615 |
| CE0036 | CHEBI:31536 | CHEMBL885 |
| CS-1370 | CTK4C4432 | Coviracil |
| Coviracil(TM) | D01199 | DB00879 |
| DRG-0208 | DSSTox_CID_20129 | DSSTox_GSID_40129 |
| DSSTox_RID_79445 | DTXSID0040129 | E1007 |
| EMTRICITABINE | EMTRIVA | EX-A150 |
| Emtricitabin | Emtricitabina | Emtricitabine |
| Emtricitabine IP Impurity D | Emtricitabine [USAN:INN] | Emtricitabinum |
| Emtritabine | Emtriva | Emtriva(TM) |
| FT-0080009 | FT-0601789 | FTC |
| FTC, (-)- | FTC-((-)) | FTC-(-) |
| G70B4ETF4S | HMS2089I05 | HMS3713L12 |
| HSDB 7337 | HY-17427 | KS-00000L28 |
| KS-00001CJ2 | LS-135838 | LS-173184 |
| MCULE-7141046266 | MFCD00870151 | MLS003882429 |
| MLS006011556 | MLS006011987 | NCGC00164564-01 |
| NE44626 | PSI 5004 | PSI-5004 |
| Q422604 | RCV | RTC-020120 |
| Racivir | SB18984 | SC-17032 |
| SCHEMBL39708 | SMR002533604 | ST2405540 |
| SW220172-1 | TC-020120 | Tox21_112193 |
| UNII-G70B4ETF4S | UNII-ULS8902U4O component XQSPYNMVSIKCOC-NTSWFWBYSA-N | W-201247 |
| W-201248 | XQSPYNMVSIKCOC-NTSWFWBYSA-N | Z1739256297 |
| ZINC3629271 | dOTFC | ent-Emtricitabine |
| s1704 |
| DrugBank Name | Emtricitabine |
| DrugBank | DB00879 |
| CAS Number | 137530-41-7, 143491-54-7, 143491-57-0, 145213-48-5 |
| PubChem Compound | 60877 |
| KEGG Compound ID | C12599 |
| KEGG Drug | D01199 |
| PubChem.Substance | 46507606 |
| ChEBI | 31536 |
| PharmGKB | PA10069 |
| ChemSpider | 54859 |
| BindingDB | 50107843.0 |
| TTD | DAP001084 |
| Wikipedia | Emtricitabine |
| HET | ETV |
| DPD | 16516 |