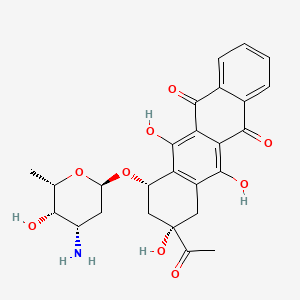
Drug
D0067 | Idarubicin
L
L01DB06 Idarubicin
[L01DB] Anthracyclines and related substances
[L01D] CYTOTOXIC ANTIBIOTICS AND RELATED SUBSTANCES
[L01] ANTINEOPLASTIC AGENTS
[L] Antineoplastic and immunomodulating agents
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| GLUCOSE GALACTOSE IC50 RATIO | 7.9 ± 3.3 , 4.2 ± 2.0, 1.9, 11.5 ± 5.7 ,6.2 ± 4.0, 1.9 | 4hr | H9c2 cells | high-glucose–galactose cell viability assay with JC-1 mitochondrial membrane potential and ATP-depletion assays (CellTiter-Glo reagent ). | glucose/galactose IC50 ratio (JC-1 IC50 in glucose, JC-1 IC50 in galactose, JC-1 glu/gla, ATP IC50 in glucose, ATP IC50 in galactose, ATP glu/gla ) | 50 | ||
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | unreported | 4900ug/kg (4.9mg/kg) | Biochemical Pharmacology. Vol. 38, Pg. 167, 1989. | |
| mouse | LD50 | oral | 16mg/kg (16mg/kg) | Cancer Treatment Reports. Vol. 61, Pg. 893, 1977. | |
| mouse | LD50 | intraperitoneal | 3mg/kg (3mg/kg) | Cancer Research. Vol. 48, Pg. 926, 1988. | |
| mouse | LD50 | intravenous | 4mg/kg (4mg/kg) | Cancer Treatment Reports. Vol. 61, Pg. 893, 1977. | |
| (1S,3S)-3-acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydronaphthacen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside | (1S,3S)-3-acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside | (7S,9S)-7-[(2R,4S,5S,6S)-4-azanyl-6-methyl-5-oxidanyl-oxan-2-yl]oxy-9-ethanoyl-6,9,11-tris(oxidanyl)-8,10-dihydro-7H-tetracene-5,12-dione |
| (7S,9S)-7-[(2R,4S,5S,6S)-4-azanyl-6-methyl-5-oxidanyl-oxan-2-yl]oxy-9-ethanoyl-6,9,11-tris(oxidanyl)-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride | (7S,9S)-9-acetyl-7-((2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yloxy)-6,9,11-trihydroxy-7,8,9,10-tetrahydrotetracene-5,12-dione | (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione |
| (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-quinone;hydrochloride | (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride | (7S,9S)-9-acetyl-7-[[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-2-oxanyl]oxy]-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione |
| (7S,9S)-9-acetyl-7-[[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-2-oxanyl]oxy]-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride | (7S,9S)-9-acetyl-7-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy}-6,9,11-trihydroxy-5,7,8,9,10,12-hexahydrotetracene-5,12-dione | 15158-EP2270008A1 |
| 15158-EP2272827A1 | 15158-EP2275420A1 | 15158-EP2277879A1 |
| 15158-EP2280012A2 | 15158-EP2281815A1 | 15158-EP2289892A1 |
| 15158-EP2292615A1 | 15158-EP2292617A1 | 15158-EP2295055A2 |
| 15158-EP2295416A2 | 15158-EP2295426A1 | 15158-EP2295427A1 |
| 15158-EP2298748A2 | 15158-EP2298764A1 | 15158-EP2298765A1 |
| 15158-EP2301928A1 | 15158-EP2301933A1 | 15158-EP2305640A2 |
| 15158-EP2305642A2 | 15158-EP2305671A1 | 15158-EP2308833A2 |
| 15158-EP2308855A1 | 15158-EP2311453A1 | 15158-EP2311808A1 |
| 15158-EP2311825A1 | 15158-EP2311827A1 | 15158-EP2311829A1 |
| 15158-EP2311842A2 | 15158-EP2316832A1 | 15158-EP2316833A1 |
| 4-DEMETHOXY-DAUNORUBICIN | 4-DMD | 4-Demethoxydaunomycin |
| 4-Demethoxydaunorubicin | 4-Demethoxydaunorubicin|||IMI-30|||NSC-256439|||(7S,9S)-9-Acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione | 4-Desmethoxydaunorubicin |
| 5,12-Naphthacenedione, 7,8,9,10-tetrahydro-9-acetyl-7-((3-amino-2,3,6-trideoxy-.alpha.-L-lyxo-hexopyranosyl)oxy)-6,9,11-trihydroxy-, (7S-cis)- | 5,12-Naphthacenedione, 7,8,9,10-tetrahydro-9-acetyl-7-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-6,9,11-trihydroxy-, (7S-cis)- | 5,12-Naphthacenedione, 9-acetyl-7-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,9,11-trihydroxy-, (7S-cis)- |
| 58957-92-9 | 957I929 | A832088 |
| AB00698511-06 | AB00698511-08 | AB00698511-09 |
| AB00698511-10 | AB00698511_11 | AC-9384 |
| AKOS015895563 | API0002970 | BCP9000773 |
| BCPP000207 | BDBM58490 | BRD-K69650333-001-01-1 |
| BRD-K69650333-001-02-9 | BRD-K69650333-003-14-0 | C-22925 |
| C26H27NO9 | CC-29462 | CCG-204689 |
| CCRIS 5083 | CHEBI:42068 | CHEMBL1117 |
| D08062 | DB01177 | DM5 |
| DTXSID7023142 | Daunomycin, 4-demethoxy- | EU-0100600 |
| FT-0621481 | GTPL7083 | HMS2089D05 |
| HMS3261H22 | I 1656 | IDARUBICIN |
| IDARUBICIN(Hydrochloride form) | IMI 30 | IMI-30 |
| Idamycin | Idarubicin (INN) | Idarubicin Hcl |
| Idarubicin [INN:BAN] | Idarubicin hydrochloride, solid | Idarubicin, United States Pharmacopeia (USP) Reference Standard |
| Idarubicina | Idarubicina [INN-Spanish] | Idarubicine |
| Idarubicine [INN-French] | Idarubicinum | Idarubicinum [INN-Latin] |
| KBioSS_002388 | LP00600 | LS-94015 |
| Lopac0_000600 | MLS001401448 | NCGC00093976-01 |
| NCGC00093976-02 | NCGC00093976-03 | NCGC00093976-04 |
| NCGC00093976-05 | NCGC00261285-01 | NSC 256439 |
| NSC-256439 | Q1063862 | SC-17016 |
| SCHEMBL3750 | SMR000466355 | SR-01000075934 |
| SR-01000075934-1 | Tox21_500600 | UNII-ZRP63D75JW |
| XDXDZDZNSLXDNA-TZNDIEGXSA-N | Z-3092 | ZINC3920266 |
| ZRP63D75JW | Zavedos (TN) | cid_636362 |
| DrugBank Name | Idarubicin |
| DrugBank | DB01177 |
| CAS Number | 57852-57-0, 58957-92-9 |
| PubChem Compound | 42890 |
| KEGG Drug | D08062 |
| PubChem.Substance | 46506973 |
| ChEBI | 42068 |
| PharmGKB | PA449961 |
| ChemSpider | 39117 |
| BindingDB | 58490.0 |
| TTD | DAP000050 |
| Wikipedia | Idarubicin |
| HET | DM5 |
| DPD | 1745 |
1. Dykens et al. (2007)