D0356 | Idebenone
N
N06BX13 Idebenone
[N06BX] Other psychostimulants and nootropics
[N06B] PSYCHOSTIMULANTS, AGENTS USED FOR ADHD AND NOOTROPICS
[N06] PSYCHOANALEPTICS
[N] Nervous system
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| ELECTRON TRANSPORT CHAIN | affect | 53 | ||||||
| ELECTRON TRANSPORT CHAIN | 0.4 μM | NADH–Q | decrease | IC50 | 106 | |||
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| NADH:ubiquinone reductase | 0.4 μM | NADH–Q | inhibitor | IC50 | 106 | |||
| quinone | antagonist | 53 | ||||||
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Warning |
Aggregated GHS information provided by 2 companies from 2 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. Reported as not meeting GHS hazard criteria by 1 of 2 companies. For more detailed information, please visit ECHA C&L website Of the 1 notification(s) provided by 1 of 2 companies with hazard statement code(s): H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
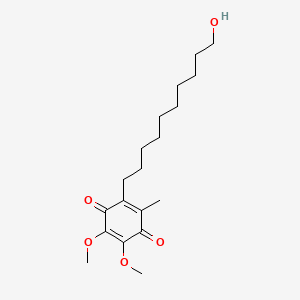
| 2-(12-hydroxydodecyl)-5,6-dimethoxy-3-methylcyclohexa-2,5-diene-1,4-dione | 6-(12-hydroxydodecyl)-2,3-dimethoxy-5-methyl-1,4-benzoquinone | DB09081 |
| SCHEMBL7407419 | VMHWZDULLBLUMS-UHFFFAOYSA-N | ZINC000137375210 |