Drug
D0439 | Bafilomycin A1
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| TRANSPORT OF PROTONS BUT NOT CHARGE (K+) | male Wistar rats | liver mitochondria | affect | 199 | ||||
| MITOPHAGY | 50 nM | SH-SY5Y neuroblastoma cells | Mitochondrial population levels were determined by flow cytometry using MitoTracker Deep Red (MTDR) dye. | 252 | ||||
| APOPTOSIS | pediatric B ALL cells | induce | 200 | |||||
| APOPTOSIS | induce | 200 | ||||||
| AUTOPHAGY | inhibit | 200 | ||||||
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| Apoptosis regulator Bcl-2 | induce binding | 200 | ||||||
| Apoptosis-inducing factor 1, mitochondrial | pediatric B ALL cells | 200 | ||||||
| Serine/threonine-protein kinase mTOR | 200 | |||||||
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Warning |
Aggregated GHS information provided by 40 companies from 3 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
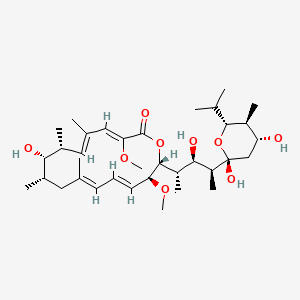
| (3Z,5E,7R,8S,9S,11E,13E,15S,16R)-16-[(1S,2R,3S)-3-[(2R,4R,5S,6R)-2,4-dihydroxy-6-isopropyl-5-methyl-tetrahydropyran-2-yl]-2-hydroxy-1-methyl-butyl]-8-hydroxy-3,15-dimethoxy-5,7,9,11-tetramethyl-1-oxacyclohexadeca-3,5,11,13-tetraen-2-one; | (3Z,5E,7R,8S,9S,11E,13E,15S,16R)-16-{(2S,3R,4S)-4-[(2R,4R,5S,6R)-2,4-dihydroxy-6-isopropyl-5-methyltetrahydro-2H-pyran-2-yl]-3-hydroxypentan-2-yl}-8-hydroxy-3,15-dimethoxy-5,7,9,11-tetramethyloxacyclohexadeca-3,5,11,13-tetraen-2-one | (3Z,5E,7R,8S,9S,11E,13E,15S,16R)-8-Hydroxy-16-[(1S,2R,3S)-2-hydroxy-1-methyl-3-[(2R,4R,5S,6R)-tetrahydro-2,4-dihydroxy-5-methyl-6-(1-methylethyl)-2H-pyran-2-yl]butyl]-3,15-dimethoxy-5,7,9,11-tetramethyloxacyclohexadeca-3,5,11,13-tetraen-2-one |
| 88899-55-2 | ABP000610 | ACon0_000813 |
| AKOS030213158 | BDBM50064186 | BSPBio_001470 |
| Bafilomycin A1(Baf-A1) | Bafilomycin A1/ | C35H58O9 |
| CHEBI:22689 | CHEMBL290814 | DB06733 |
| HMS3402J12 | Hygrolidin, 21-O-de(3-carboxy-1-oxo-2-propenyl)-2-demethyl-2-methoxy-24-methyl- | LS-178068 |
| MCULE-2359469972 | MEGxm0_000385 | MFCD06795130 |
| NCGC00163426-02 | NSC 381866 | NSC381866 |
| Q4841341 | SCHEMBL13775181 | ZINC169647947 |
| bafilomycin A1 |
| DrugBank Name | Bafilomycin A1 |
| DrugBank | DB06733 |
| CAS Number | 88899-55-2 |
| PubChem Compound | 6436223 |
| ChEBI | 22689 |
| ChemSpider | 10251049 |
| Wikipedia | Bafilomycin |