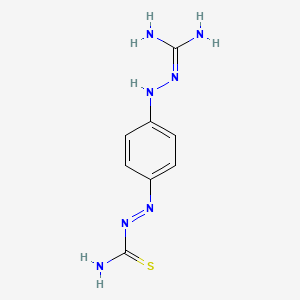
D0773 | ambazone
R
R02AA01 Ambazone
[R02AA] Antiseptics
[R02A] THROAT PREPARATIONS
[R02] THROAT PREPARATIONS
[R] Respiratory system
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| MEMBRANE POTENTIAL | 5.60±3.23 | human | qHTS-HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 3.98 | human | HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 1.98±2.22 | rat | hepatocytes | MMP assay | decrease | IC50 | 163 | |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Warning |
Aggregated GHS information provided by 2 companies from 2 notifications to the ECHA C&L Inventory. Reported as not meeting GHS hazard criteria by 1 of 2 companies. For more detailed information, please visit ECHA C&L website Of the 1 notification(s) provided by 1 of 2 companies with hazard statement code(s): H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P264, P270, P301+P312, P330, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| ((4-Oxo-2,5-cyclohexadien-1-ylidene)amino)guanidine thiosemicarbazone | (2E)-2-[(4E)-4-(2-Carbamimidoylhydrazinylidene)cyclohexa-2,5-dien-1-ylidene]hydrazine-1-carbothioamide | (2E)-2-{(4E)-4-[(aminocarbonothioyl)hydrazono]cyclohexa-2,5-dien-1-ylidene}hydrazinecarboximidamide |
| (2Z)-2-[(4Z)-4-(2-carbamimidoylhydrazinylidene)cyclohexa-2,5-dien-1-ylidene]hydrazinecarbothioamide | 1,4-Benzoquinone guanylhydrazone thiosemicarbazone | 1,4-benzoquinoneguanylhydrazonethiosemicarbazone |
| 1-Amidinohydrazono-4-thiosemicarbazono-2,5-cyclohexadiene | 1-[4-[2-[bis(azanyl)methylidene]hydrazinyl]phenyl]iminothiourea | 4-Amidinohydrazono-2,5-cyclohexadien-1-on-thiosemicarbazon |
| 539-21-9 | 539A219 | AKOS000622836 |
| AKOS002161802 | AKOS005197779 | API0010662 |
| Ambazon | Ambazona | Ambazona [INN-Spanish] |
| Ambazone | Ambazone (INN) | Ambazone [INN:BAN:DCF] |
| Ambazonum | Ambazonum [INN-Latin] | Anginon |
| BDBM53352 | BYK4592A3Q | Benzoquinone guanylhydrazone thiosemicarbazone |
| C8H11N7S | CAS-539-21-9 | CCRIS 1926 |
| CHEBI:134962 | CHEMBL2103762 | CHEMBL3198213 |
| D07376 | DB13697 | DC 0572 |
| DSSTox_CID_26407 | DSSTox_GSID_46407 | DSSTox_RID_81587 |
| DTXSID3046407 | EINECS 208-713-0 | FT-0661544 |
| Faringosept | Faringosept (TN) | Guanidine, ((4-oxo-2,5-cyclohexadien-1-ylidene)amino)-, thiosemicarbazone |
| Guanothiazon | HMS2231L21 | HYD014 |
| Hydrazinecarbothioamide, 2-(4-((aminoiminomethyl)hydrazono)-2,5-cyclohexadien-1-ylidene)- | Hydrazinecarbothioamide, 2-[4-[(aminoiminomethyl)hydrazono]-2,5-cyclohexadien-1-ylidene] | Inversal |
| Iversal | Ivertol | LS-73851 |
| MCULE-8433703539 | MLS001240207 | N''-{(1Z,4Z)-4-[(aminocarbonothioyl)hydrazono]cyclohexa-2,5-dien-1-ylidene}carbonohydrazonic diamide; |
| NCGC00164514-01 | NCGC00164514-02 | Primal |
| Promassol | Q414317 | SCHEMBL11887782 |
| SCHEMBL1649608 | SCHEMBL457920 | SMR000674566 |
| SR-01000799157 | SR-01000799157-2 | STL200147 |
| Tox21_112149 | UNII-BYK4592A3Q | ZINC1550965 |
| [4-(2-(diaminomethylidene)hydrazino)phenyl]iminothiourea | [4-[2-(diaminomethylidene)hydrazinyl]phenyl]iminothiourea | [4-[N''-(diaminomethylene)hydrazino]phenyl]iminothiourea |
| cid_1549158 | p-Benzoquinone amidinohydrazone thiosemicarbazone | {[4-(carbamimidamidoimino)cyclohexa-2,5-dien-1-ylidene]amino}thiourea |