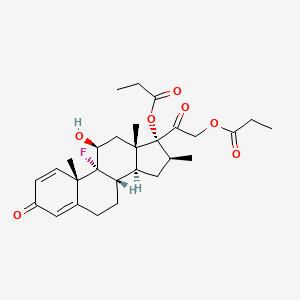
D0802 | betamethasone dipropionate
D
S
A
H
R
C
S03CA06 Betamethasone and antiinfectives
[S03CA] Corticosteroids and antiinfectives in combination
[S03C] CORTICOSTEROIDS AND ANTIINFECTIVES IN COMBINATION
[S03] OPHTHALMOLOGICAL AND OTOLOGICAL PREPARATIONS
[S] Sensory organs
S03BA03 Betamethasone
[S03BA] Corticosteroids
[S03B] CORTICOSTEROIDS
[S03] OPHTHALMOLOGICAL AND OTOLOGICAL PREPARATIONS
[S] Sensory organs
S02BA07 Betamethasone
[S02BA] Corticosteroids
[S02B] CORTICOSTEROIDS
[S02] OTOLOGICALS
[S] Sensory organs
S01CB04 Betamethasone
[S01CB] Corticosteroids/antiinfectives/mydriatics in combination
[S01C] ANTIINFLAMMATORY AGENTS AND ANTIINFECTIVES IN COMBINATION
[S01] OPHTHALMOLOGICALS
[S] Sensory organs
S01CA05 Betamethasone and antiinfectives
[S01CA] Corticosteroids and antiinfectives in combination
[S01C] ANTIINFLAMMATORY AGENTS AND ANTIINFECTIVES IN COMBINATION
[S01] OPHTHALMOLOGICALS
[S] Sensory organs
S01BB04 Betamethasone and mydriatics
[S01BB] Corticosteroids and mydriatics in combination
[S01B] ANTIINFLAMMATORY AGENTS
[S01] OPHTHALMOLOGICALS
[S] Sensory organs
S01BA06 Betamethasone
[S01BA] Corticosteroids, plain
[S01B] ANTIINFLAMMATORY AGENTS
[S01] OPHTHALMOLOGICALS
[S] Sensory organs
R03BA04 Betamethasone
[R03BA] Glucocorticoids
[R03B] OTHER DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES, INHALANTS
[R03] DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES
[R] Respiratory system
R01AD06 Betamethasone
[R01AD] Corticosteroids
[R01A] DECONGESTANTS AND OTHER NASAL PREPARATIONS FOR TOPICAL USE
[R01] NASAL PREPARATIONS
[R] Respiratory system
H02AB01 Betamethasone
[H02AB] Glucocorticoids
[H02A] CORTICOSTEROIDS FOR SYSTEMIC USE, PLAIN
[H02] CORTICOSTEROIDS FOR SYSTEMIC USE
[H] Systemic hormonal preparations, excluding reproductive hormones and insulins
D07XC01 Betamethasone
[D07XC] Corticosteroids, potent, other combinations
[D07X] CORTICOSTEROIDS, OTHER COMBINATIONS
[D07] CORTICOSTEROIDS, DERMATOLOGICAL PREPARATIONS
[D] Dermatological drugs
D07CC01 Betamethasone and antibiotics
[D07CC] Corticosteroids, potent, combinations with antibiotics
[D07C] CORTICOSTEROIDS, COMBINATIONS WITH ANTIBIOTICS
[D07] CORTICOSTEROIDS, DERMATOLOGICAL PREPARATIONS
[D] Dermatological drugs
D07BC01 Betamethasone and antiseptics
[D07BC] Corticosteroids, potent, combinations with antiseptics
[D07B] CORTICOSTEROIDS, COMBINATIONS WITH ANTISEPTICS
[D07] CORTICOSTEROIDS, DERMATOLOGICAL PREPARATIONS
[D] Dermatological drugs
D07AC01 Betamethasone
[D07AC] Corticosteroids, potent (group III)
[D07A] CORTICOSTEROIDS, PLAIN
[D07] CORTICOSTEROIDS, DERMATOLOGICAL PREPARATIONS
[D] Dermatological drugs
C05AA05 Betamethasone
[C05AA] Corticosteroids
[C05A] AGENTS FOR TREATMENT OF HEMORRHOIDS AND ANAL FISSURES FOR TOPICAL USE
[C05] VASOPROTECTIVES
[C] Cardiovascular system
A07EA04 Betamethasone
[A07EA] Corticosteroids acting locally
[A07E] INTESTINAL ANTIINFLAMMATORY AGENTS
[A07] ANTIDIARRHEALS, INTESTINAL ANTIINFLAMMATORY/ANTIINFECTIVE AGENTS
[A] Alimentary tract and metabolism
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| MEMBRANE POTENTIAL | 25.60±1.67 | human | qHTS-HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 19.45 | human | HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | rat | hepatocytes | MMP assay | Negative | IC50 | 163 | ||
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
   |
Danger |
Aggregated GHS information provided by 108 companies from 10 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. Reported as not meeting GHS hazard criteria by 2 of 108 companies. For more detailed information, please visit ECHA C&L website Of the 9 notification(s) provided by 106 of 108 companies with hazard statement code(s): H330 (33.02%): Fatal if inhaled [Danger Acute toxicity, inhalation] H360 (44.34%): May damage fertility or the unborn child [Danger Reproductive toxicity] H361 (54.72%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] H372 (42.45%): Causes damage to organs through prolonged or repeated exposure [Danger Specific target organ toxicity, repeated exposure] H410 (42.45%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P260, P264, P270, P271, P273, P281, P284, P304+P340, P308+P313, P310, P314, P320, P391, P403+P233, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | subcutaneous | > 4gm/kg (4000mg/kg) | Shikoku Igaku Zasshi. Shikoku Medical Journal. Vol. 29, Pg. 153, 1973. | |
| rat | LD50 | intraperitoneal | > 4gm/kg (4000mg/kg) | Shikoku Igaku Zasshi. Shikoku Medical Journal. Vol. 29, Pg. 153, 1973. | |
| mouse | LD50 | intraperitoneal | 103mg/kg (103mg/kg) | Drugs in Japan Vol. 6, Pg. 753, 1982. | |
| rat | LD50 | oral | > 4gm/kg (4000mg/kg) | Shikoku Igaku Zasshi. Shikoku Medical Journal. Vol. 29, Pg. 153, 1973. | |
| mouse | LD50 | subcutaneous | 78100ug/kg (78.1mg/kg) | Drugs in Japan Vol. 6, Pg. 753, 1982. | |
| mouse | LD50 | oral | > 5gm/kg (5000mg/kg) | Shikoku Igaku Zasshi. Shikoku Medical Journal. Vol. 29, Pg. 153, 1973. | |
| (11-beta,16-beta)-9-Chloro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione | (11.beta.,16.beta.)-9-Fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate | (11beta,16beta)-9-fluoro-11-hydroxy-16-methyl-3,20-dioxopregna-1,4-diene-17,21-diyl dipropanoate |
| (8S,9R,10S,11S,13S,14S,16S,17R)-9-fluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-17-(2-(propionyloxy)acetyl)-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthr | 2-((2S,10S,11S,13S,15S,17S,1R,14R)-1-fluoro-17-hydroxy-2,13,15-trimethyl-5-oxo -14-propanoyloxytetracyclo[8.7.0.0<2,7>.0<11,15>]heptadeca-3,6-dien-14-yl)-2-o xoethyl propanoate | 5593-20-4 |
| 826Y60901U | 9-Fluoro-11.beta.,17,21-trihydroxy-16.beta.-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate | 9-Fluoro-11.beta.-hydroxy-16.beta.-methyl-3,20-dioxo-17-(propionyloxy)pregna-1,4-dien-21-yl propionate # |
| 9-Fluoro-11beta,17,21-trihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 17,21-di(propionate) | 9-Fluoro-11beta,17,21-trihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate | 9-Fluoro-11beta,17,21-trihydroxy-16beta-methylpregna-1,4-diene-3,20-dione-17,21-dipropionate |
| 9-fluoro-11beta-hydroxy-16beta-methyl-3,20-dioxopregna-1,4-diene-17,21-diyl dipropanoate | 9alpha-Fluoro-11beta,17alpha,21-trihydroxy-16beta-methyl-1,4-pregnadiene-3,20-dione 17,21-Dipropionate | 9alpha-fluoro-11beta,17alpha,21-trihydroxy-16beta-methyl-3,20-dioxopregna-1,4-diene- 17,21-diyl dipropionate |
| AB01274713-01 | AB01274713_02 | AB2000220 |
| AKOS015969733 | Alphatrex | B3166 |
| BDBM50421892 | BETAMETHASONE DIPROP | BETAMETHASONE DIPROPIONATE |
| BRD-K58148589-001-03-6 | BRN 3638108 | Beloderm |
| Betamethasone 17,21-dipropionate | Betamethasone Dipropionate (Diprolene) | Betamethasone dipropionate (JP17/USP) |
| Betamethasone dipropionate [USAN:BAN:JAN] | Betamethasone dipropionate [USAN:USP:JAN] | Betamethasone-17,21-dipropionate |
| Betamethasone-Dipropionate(Diprolene) | C-22966 | C28H37FO7 |
| CAS-5593-20-4 | CC-24614 | CHEBI:31276 |
| CHEMBL1200384 | CIWBQSYVNNPZIQ-XYWKZLDCSA-N | CS-7549 |
| D01637 | DSSTox_CID_2672 | DSSTox_GSID_22672 |
| DSSTox_RID_76683 | DTXSID2022672 | Diproderm |
| Diprolene | Diprolene (TN) | Diprolene AF |
| Diprosis | Diprosone | Diprospan;Diprolene;Diprosone;Diprolene AF;Diprolene |
| EBD2157850 | EINECS 227-005-2 | HY-13571 |
| KS-5303 | LS-118474 | Maxivate |
| NCGC00159360-02 | NCGC00159443-01 | NCGC00159443-02 |
| Pregna-1,4-diene-3,20-dione, 9-fluoro-11-.beta.,17,21-trihydroxy-16-.beta.-methyl-,17,21-dipropionate | Pregna-1,4-diene-3,20-dione, 9-fluoro-11-beta,17,21-trihydroxy-16-beta-methyl-, 17,21-dipropionate | Pregna-1,4-diene-3,20-dione, 9-fluoro-11-beta,17,21-trihydroxy-16-beta-methyl-,17,21-dipropionate; |
| Pregna-1,4-diene-3,20-dione, 9-fluoro-11-hydroxy-16-methyl-17,21-bis(1-oxopropoxy)-, (11.beta.,16.beta.)- | Pregna-1,4-diene-3,20-dione, 9-fluoro-11-hydroxy-16-methyl-17,21-bis(1-oxopropoxy)-, (11beta,16beta) | Psorion |
| Q4897349 | Rinderon DP | Rinderon-DP (TN) |
| S 3440 | S-3440 | SCHEMBL7519 |
| ST024761 | Sch 11460 | Sch-11460 |
| Sernivo | Sernivo (TN) | Tox21_113343 |
| UNII-826Y60901U | ZINC4212137 | [2-[(8S,9R,10S,11S,13S,14S,16S,17R)-9-fluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-17-propanoyloxy-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl]-2-oxoethyl] propanoate |
| beta-Methasone 17,21-dipropionate | betamethasone dipropionat | betamethasone propionate |
| component of Alphatrex (Salt/Mix) | component of Betasone (Salt/Mix) | en-17-yl propionate |
| s1688 |