Drug
D0815 | bromocriptine
G
N
N04BC01 Bromocriptine
[N04BC] Dopamine agonists
[N04B] DOPAMINERGIC AGENTS
[N04] ANTI-PARKINSON DRUGS
[N] Nervous system
G02CB01 Bromocriptine
[G02CB] Prolactine inhibitors
[G02C] OTHER GYNECOLOGICALS
[G02] OTHER GYNECOLOGICALS
[G] Genitourinary system and reproductive hormones
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| MEMBRANE POTENTIAL | 11.44±1.58 | human | qHTS-HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 21.82 | human | HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 13.20±10.83 | rat | hepatocytes | MMP assay | decrease | IC50 | 163 | |
| MITOCHONDRIA TRANSPORT | 5 µM | 2h | rat | Hippocampal Neurons | time-lapse imaging experiments | decrease | 216 | |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Warning |
The GHS information provided by 1 company from 1 notification to the ECHA C&L Inventory. H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] H361 (100%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] H362 (100%): May cause harm to breast-fed children [Reproductive toxicity, effects on or via lactation] |
P201, P202, P260, P263, P264, P270, P281, P301+P312, P308+P313, P330, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| women | TDLo | oral | 650ug/kg/9D-I (0.65mg/kg) | Annals of Internal Medicine. Vol. 118, Pg. 199, 1993. | |
| rat | LD50 | intravenous | 10500ug/kg (10.5mg/kg) | Yakkyoku. Pharmacy. Vol. 29, Pg. 1231, 1978. | |
| mouse | LD50 | intravenous | 189mg/kg (189mg/kg) | Yakkyoku. Pharmacy. Vol. 30, Pg. 809, 1979. | |
| child | TDLo | oral | 375ug/kg (0.375mg/kg) | Journal of Pediatrics. Vol. 105, Pg. 838, 1984. | |
| man | TDLo | oral | 52mg/kg/35W-I (52mg/kg) | brain and coverings: changes in cerebral spinal fluid | Neurology. Vol. 35, Pg. 1193, 1985. |
| rat | LD50 | oral | > 2gm/kg (2000mg/kg) | Yakkyoku. Pharmacy. Vol. 30, Pg. 809, 1979. | |
| rabbit | LD50 | intravenous | 8200ug/kg (8.2mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 10, Pg. 232, 1979. | |
| women | TDLo | oral | 50ug/kg (0.05mg/kg) | sense organs and special senses: other: eye | Lancet. Vol. 340, Pg. 1410, 1992. |
| women | TDLo | oral | 1mg/kg/20D-I (1mg/kg) | behavioral: toxic psychosis | American Journal of Psychiatry. Vol. 143, Pg. 935, 1985. |
| mouse | LD50 | oral | 2502mg/kg (2502mg/kg) | Yakkyoku. Pharmacy. Vol. 30, Pg. 809, 1979. | |
| rat | LD50 | subcutaneous | > 1gm/kg (1000mg/kg) | Yakkyoku. Pharmacy. Vol. 30, Pg. 809, 1979. | |
| rabbit | LD50 | oral | > 1gm/kg (1000mg/kg) | Yakkyoku. Pharmacy. Vol. 30, Pg. 809, 1979. | |
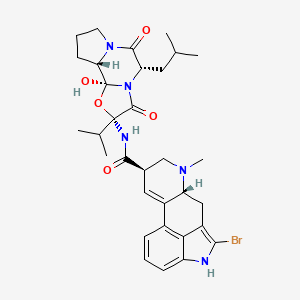
| (4R,7R)-10-bromo-N-[(1S,2S,4R,7S)-2-hydroxy-7-(2-methylpropyl)-5,8-dioxo-4-(propan-2-yl)-3-oxa-6,9-diazatricyclo[7.3.0.0^{2,6}]dodecan-4-yl]-6-methyl-6,11-diazatetracyclo[7.6.1.0^{2,7}.0^{12,16}]hexadeca-1(16),2,9,12,14-pentaene-4-carboxamide | (5'alpha)-2-bromo-12'-hydroxy-2'-(1-methylethyl)-5'-(2-methylpropyl)-3',6',18-trioxoergotaman | (5'alpha)-2-bromo-12'-hydroxy-2'-(1-methylethyl)-5'-(2-methylpropyl)ergotaman-3',6',18-trione |
| (5'alpha)-2-bromo-12'-hydroxy-5'-(2-methylpropyl)-2'-(propan-2-yl)-3',6',18-trioxoergotaman | (5'alpha)-2-bromo-12'-hydroxy-5'-(2-methylpropyl)-3',6',18-trioxo-2'-(propan-2-yl)ergotaman | (5'alpha)-2-bromo-12'-hydroxy-5'-isobutyl-2'-isopropyl-3',6',18-trioxoergotaman |
| (5alpha,5'beta)-2-bromo-12'-hydroxy-5'-(2-methylpropyl)-3',6',18-trioxo-2'-(propan-2-yl)ergotaman | (6aR,9R)-5-Bromo-N-((2R,5S,10aS,10bS)-10b-hydroxy-5-isobutyl-2-isopropyl-3,6-dioxooctahydro-2H-oxazolo[3,2-a]pyrrolo[2,1-c]pyrazin-2-yl)-7-methyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]quinoline-9-carboxamide | (6aR,9R)-5-bromo-N-((2R,5S,10aS,10bS)- |
| 08Y | 10b-hydroxy-5-isobutyl-2-isopropyl-3,6- | 13177-EP2272832A1 |
| 13177-EP2275420A1 | 13177-EP2277876A1 | 13177-EP2280008A2 |
| 13177-EP2287165A2 | 13177-EP2287166A2 | 13177-EP2292614A1 |
| 13177-EP2292620A2 | 13177-EP2295412A1 | 13177-EP2295413A1 |
| 13177-EP2295439A1 | 13177-EP2298731A1 | 13177-EP2298738A1 |
| 13177-EP2298779A1 | 13177-EP2311808A1 | 13177-EP2311818A1 |
| 13177-EP2311829A1 | 13177-EP2311837A1 | 13177-EP2316470A2 |
| 13177-EP2316834A1 | 2-Bromo-12'-hydroxy-2'-(1-methylethyl)-5'-alpha-(2-methylpropyl)ergotamin-3',6',18-trione | 2-Bromo-alpha-ergocryptine |
| 2-Bromo-alpha-ergokryptin | 2-Bromo-alpha-ergokryptine | 2-Bromoergocryptine |
| 2-Bromoergocryptine Methanesulfonate | 2-Bromoergokryptine | 25614-03-3 |
| 3A64E3G5ZO | 88085-EP2298415A1 | 88085-EP2298742A1 |
| 88085-EP2305648A1 | AC-13601 | AKOS015961273 |
| BDBM81993 | BIDD:GT0464 | BPBio1_001131 |
| BRD-K14496212-001-01-1 | BRD-K14496212-066-04-8 | Biomol-NT_000005 |
| Bromergocryptine | Bromocriptin | Bromocriptina |
| Bromocriptina [INN-Spanish] | Bromocriptine (USAN/INN) | Bromocriptine [BAN] |
| Bromocriptine [USAN:BAN:INN] | Bromocriptine [USAN:INN:BAN] | Bromocriptine methanesulfonate |
| Bromocriptine+ (GTP-) | Bromocriptinum | Bromocriptinum [INN-Latin] |
| Bromocryptin | Bromocryptine | Bromoergocriptine |
| Bromoergocryptine | C06856 | C32H40BrN5O5 |
| CAS-25614-03-3 | CB-154 | CCG-204266 |
| CCRIS 3244 | CHEBI:3181 | CHEMBL493 |
| Carboprost Methylate,(S) | D03165 | DB01200 |
| DSSTox_CID_2687 | DSSTox_GSID_22687 | DSSTox_RID_76692 |
| DTXSID1022687 | EINECS 247-128-5 | Ergocryptine, 2-bromo- |
| Ergocryptine, 2-bromo- (8CI) | Ergoset | Ergotaman-3',6',18-trione, 2-bromo-12'-hydroxy-2'-(1-methylethyl)-5'-(2-methylpropyl)-, (5'alpha)- |
| Ergotaman-3',6',18-trione, 2-bromo-12'-hydroxy-2'-(1-methylethyl)-5'-(2-methylpropyl)-,(5'-alpha)- | Ergotaman-3',6',18-trione, 2-bromo-12'-hydroxy-2'-(1-methylethyl)-5'alpha-(2-methylpropyl)- | GTPL35 |
| J-016067 | LS-64540 | Lopac0_000171 |
| N-[(2R,5S,10aS,10bS)-10b-hydroxy-5-isobutyl-2-isopropyl-3,6-dioxo-8,9,10,10a-tetrahydro-5H-oxazolo[[?]]pyrrolo[[?]]pyrazin-2-yl]-bromo-methyl-[?]carboxamide; | NCGC00024584-03 | NCGC00024584-04 |
| NCGC00024584-05 | NCI60_001365 | NSC169774 |
| OZVBMTJYIDMWIL-AYFBDAFISA-N | PDSP2_001500 | Parlodel |
| Prestwick0_000121 | Prestwick1_000121 | Prestwick2_000121 |
| Q413581 | SANDOZ 15-754 | SCHEMBL25297 |
| SPBio_002101 | SR-01000075356 | SR-01000075356-5 |
| Tox21_110907 | UNII-3A64E3G5ZO | ZINC53683151 |
| [2,1-c]pyrazin-2-yl)-7-methyl-4,6,6a,7,8,9- | bromocriptine | dioxooctahydro-2H-oxazolo[3,2-a]pyrrolo |
| hexahydroindolo[4,3-fg]quinoline-9-carboxamide |