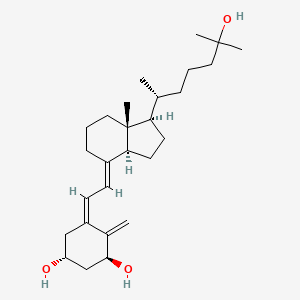
D0832 | calcitriol
D
A
D05AX03 Calcitriol
[D05AX] Other antipsoriatics for topical use
[D05A] ANTIPSORIATICS FOR TOPICAL USE
[D05] ANTIPSORIATICS
[D] Dermatological drugs
A11CC04 Calcitriol
[A11CC] Vitamin D and analogues
[A11C] VITAMIN A AND D, INCL. COMBINATIONS OF THE TWO
[A11] VITAMINS
[A] Alimentary tract and metabolism
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| MEMBRANE POTENTIAL | 14.06±4.15 | human | qHTS-HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 15.45 | human | HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 22.41±7.17 | rat | hepatocytes | MMP assay | decrease | IC50 | 163 | |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Danger |
Aggregated GHS information provided by 89 companies from 10 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H300 (100%): Fatal if swallowed [Danger Acute toxicity, oral] H310 (96.63%): Fatal in contact with skin [Danger Acute toxicity, dermal] H330 (49.44%): Fatal if inhaled [Danger Acute toxicity, inhalation] H361 (98.88%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P260, P262, P264, P270, P271, P280, P281, P284, P301+P310, P302+P350, P304+P340, P308+P313, P310, P320, P321, P322, P330, P361, P363, P403+P233, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 1900ug/kg (1.9mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 11, Pg. 4175, 1983. | |
| rat | LD50 | intravenous | 105ug/kg (0.105mg/kg) | Japanese Kokai Tokyo Koho Patents. Vol. #94-247858, | |
| rat | LD50 | intraperitoneal | > 5mg/kg (5mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 11, Pg. 4175, 1983. | |
| rat | LD50 | subcutaneous | 66ug/kg (0.066mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 11, Pg. 4175, 1983. | |
| mouse | LD50 | oral | 1350ug/kg (1.35mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 11, Pg. 4175, 1983. | |
| mouse | LD50 | subcutaneous | 145ug/kg (0.145mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 11, Pg. 4175, 1983. | |
| rat | LD50 | oral | 620ug/kg (0.62mg/kg) | Japanese Kokai Tokyo Koho Patents. Vol. #94-247858, | |
| (1?,3?,5Z,7E)-9,10-Secocholesta-5,7,10(19)-triene-1,3,25-triol | (1R,3S)-5-{2-[(1R,3aS,7aR)-1-((R)-5-Hydroxy-1,5-dimethyl-hexyl)-7a-methyl-octahydro-inden-4-ylidene]-ethylidene}-4-methylene-cyclohexane-1,3-diol | (1R,3S,5Z)-5-[(2E)-2-[(1R,3aS,7aR)-1-[(1R)-5-hydroxy-1,5-dimethyl-hexyl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-4-methylene-cyclohexane-1,3-diol |
| (1R,3S,5Z)-5-[(2E)-2-[(1R,3aS,7aR)-1-[(2R)-6-hydroxy-6-methylheptan-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-4-methylidenecyclohexane-1,3-diol | (1R,3S,Z)-5-((E)-2-((1R,3aS,7aR)-1-((R)-6-hydroxy-6-methylheptan-2-yl)-7a-methylhexahydro-1H-inden-4(2H)-ylidene)ethylidene)-4-methylenecyclohexane-1,3-diol | (1R,3S,Z)-5-(2-((1R,3aS,7aR,E)-1-((R)-6-hydroxy-6-methylheptan-2-yl)-7a-methyloctahydro-4H-inden-4-ylidene)ethylidene)-4-methylenecyclohexane-1,3-diol; |
| (1S,3R,5Z,7E)-9,10-seco-5,7,10(19)-cholestatriene-1,3,25-triol | (1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-1,3,25-triol | (1S,3R,5Z,7E)-9,10-secocholesta-5,7,10-triene-1,3,25-triol |
| (1S,3R,5Z,7E,14beta,17alpha)-9,10-secocholesta-5,7,10-triene-1,3,25-triol | (1alpha,3beta,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-1,3,25-triol | (3b,5Z,7E)-9,10-Secocholesta-5,7,10(19)-trienetriol |
| (5Z,10-secocholesta-5,7,10(19)-triene-1.alpha.,3.beta.,25-triol | (5Z,7E)-(1S,3R)-9,10-seco-5,7,10(19)-cholestatriene-1,3,25-triol | (5Z,7E)-(1S,3R)-9,10-secocholesta-5,7,10(19)-triene-1,3,25-triol |
| (5Z,7E)-9,10-Secocholesta-5,7,10(19)-triene-1-alpha,3-beta,25-triol | (5Z,7E)-9,10-Secocholesta-5,7,10(19)-triene-1alpha,3beta,25-triol | 1 alpha,25-Dihydroxyvitamin D3 |
| 1,25 (OH)2 D3 | 1,25 DIHYDROXY VITAMIN D3 | 1,25 Dihydroxycholecalciferol |
| 1,25(OH)2-20epi-D3 | 1,25(OH)2D3 & CD4 | 1,25(OH2)D3 |
| 1,25-(OH)2-D3 | 1,25-(OH)2D3 | 1,25-DHCC |
| 1,25-DIHYDROXYCHOLECALCIFEROL | 1,25-Dihydroxycholecaliferol | 1,25-Dihydroxyvitamin D |
| 1,25-Dihydroxyvitamin D3 | 1,25-dihydroxy vitamin D3 | 1,25-dihydroxy-20-epi-Vitamin D3 |
| 1,25D3 | 1-alpha,-1,25-Dihydroxyvitamin D3 | 1-alpha,25-Dihydroxycholecalciferol |
| 1-alpha,25-Dihydroxyvitamin D3 | 1-alpha-25-dihydroxyvitamin D3 | 1.Alpha.,25-Dihydroxy-26,27-hexadeuterovitamin D3 |
| 1.alpha.,25-Dihydroxyvitamin D(sub 3) | 1.alpha.,25-dihydroxycholecalciferol | 1a,25-(OH)2D3 |
| 1a,25-Dihydroxycholecalciferol | 1a,25-Dihydroxyvitamin D3 | 1alpha 25-dihydroxycholecalciferol |
| 1alpha,25(OH)2-D3 | 1alpha,25(OH)2D3 | 1alpha,25-Dihydroxycholecalciferol |
| 1alpha,25-Dihydroxyvitamin D | 1alpha,25-Dihydroxyvitamin D3 | 1alpha,25-Dihydroxyvitamin D3, >=97.0% (HPLC) |
| 1alpha,25-Dihydroxyvitamin D3, >=99% (HPLC) | 1alpha,25-dihydroxyvitamin D3 / 1alpha,25-dihydroxycholecalciferol / calcitriol | 1db1 |
| 1|A,25-Dihydroxyvitamin D3 | 20-epi-1alpha,25-dihydroxycholecaliferol | 222C063 |
| 25-dihydroxycholecalciferol | 32222-06-3 | 5-{2-[1-(5-HYDROXY-1,5-DIMETHYL-HEXYL)-7A-METHYL-OCTAHYDRO-INDEN-4-YLIDENE]-ETHYLIDENE}-4-METHYLENE-CYCLOHEXANE-1,3-DIOL |
| 62019-EP2292592A1 | 62019-EP2298772A1 | 62019-EP2308828A2 |
| 62019-EP2308839A1 | 9,10-Seco(5Z,7E)-5,7,10(19)-cholestatriene-1alpha,3beta,25-triol | 9,10-Secocholesta-5,7,10(19)-triene-1,3,25-triol, (1.alpha.,3.beta,.5Z,7E)- |
| 9,10-Secocholesta-5,7,10(19)-triene-1,3,25-triol, (1.alpha.,3.beta,.5Z,7E)- & CD4 | 9,10-Secocholesta-5,7,10(19)-triene-1,3,25-triol, (1alpha,3beta,5Z,7E)- | 9,10-seco(5Z,7E)-5,7,10(19)-cholestatriene-1alpha, 3beta, 25-triol |
| AB00639957-06 | AB00639957_07 | AC-1859 |
| ACT06832 | AKOS015961898 | Asentar |
| BCBcMAP01_000160 | BCP9000474 | BCPP000304 |
| BDBM50200182 | BML2-E03 | BRD-K27316855-001-06-7 |
| BRD-K27316855-001-19-0 | BSPBio_001287 | C01673 |
| CAS-32222-06-3 | CCG-101001 | CCRIS 5522 |
| CD-2027 | CHEBI:17823 | CHEBI:93988 |
| CHEMBL846 | CPD000466393 | CS-0388 |
| Calcijex | Calcitriol (JAN/USP/INN) | Calcitriol (Rocaltrol) |
| Calcitriol [USAN:INN:BAN:JAN] | Calcitriol [USAN:USP:INN:BAN:JAN] | Calcitriol, European Pharmacopoeia (EP) Reference Standard |
| Calcitriol-(Rocaltrol) | Calcitriolum | Calcitriolum [INN-Latin] |
| Cholecalciferol, 1-alpha,25-dihydroxy- | D00129 | DB00136 |
| DN 101 | DN-101 | DSSTox_CID_2722 |
| DSSTox_GSID_22722 | DSSTox_RID_76700 | DTXSID5022722 |
| Decostriol | Dihydroxyvitamin D3 | EINECS 250-963-8 |
| FXC9231JVH | GMRQFYUYWCNGIN-NKMMMXOESA-N | GTPL2779 |
| HMS1361A09 | HMS1791A09 | HMS1989A09 |
| HMS2051F06 | HMS2089N03 | HMS2232D18 |
| HMS3402A09 | HSDB 3482 | HY-10002 |
| IDI1_033757 | LMST03020258 | LS-53093 |
| MC-1288 | MC1288 | MFCD00867079 |
| MLS000759536 | MLS001424122 | NC00251 |
| NCGC00161327-01 | NCGC00161327-04 | NSC-749776 |
| NSC749776 | Panbonis | PubChem18818 |
| PubChem19327 | Q139195 | RO215535 |
| Ro 21-5535 | Ro 215535 | Ro-21-5535 |
| Ro-215535 | Rocaltrol | Rocaltrol (TN) |
| SC-17020 | SCHEMBL3245 | SMR000466393 |
| SR-01000759361 | SR-01000759361-4 | SR-01000946978 |
| SR-01000946978-1 | Silkis | Soltriol |
| Spectrum5_002061 | Synthetic vitamin D analog | Topitriol |
| Toptriol | Tox21_111988 | U 49562 |
| UNII-FXC9231JVH | Vectical | Vitamin D3, 1alpha, 25-Dihydroxy- |
| W-5161 | ZINC100015048 | ZX-AFC000310 |
| calcitriol | dihydroxy-vitamin D3 | s1466 |
| vit D |