D0851 | clobetasol
D
D07CD01 Clobetasol and antibiotics
[D07CD] Corticosteroids, very potent, combinations with antibiotics
[D07C] CORTICOSTEROIDS, COMBINATIONS WITH ANTIBIOTICS
[D07] CORTICOSTEROIDS, DERMATOLOGICAL PREPARATIONS
[D] Dermatological drugs
D07AD01 Clobetasol
[D07AD] Corticosteroids, very potent (group IV)
[D07A] CORTICOSTEROIDS, PLAIN
[D07] CORTICOSTEROIDS, DERMATOLOGICAL PREPARATIONS
[D] Dermatological drugs
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| MEMBRANE POTENTIAL | 14.40±3.67 | human | qHTS-HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 17.34 | human | HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 19.76±15.65 | rat | hepatocytes | MMP assay | decrease | IC50 | 163 | |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Danger |
Aggregated GHS information provided by 41 companies from 4 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H360 (92.68%): May damage fertility or the unborn child [Danger Reproductive toxicity] H373 (97.56%): Causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure] H413 (92.68%): May cause long lasting harmful effects to aquatic life [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P260, P273, P281, P308+P313, P314, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
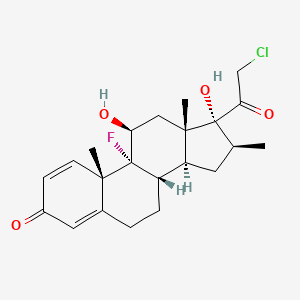
| (11beta,16beta)-21-chloro-9-fluoro-11,17-dihydroxy-16-methylpregna-1,4-diene-3,20-dione | (1R,2S,10S,11S,13S,14R,15S,17S)-14-(2-chloroacetyl)-1-fluoro-14,17-dihydroxy-2,13,15-trimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-3,6-dien-5-one | (8S,9R,10S,11S,13S,14S,16S,17R)-17-(2-chloroacetyl)-9-fluoro-11,17-dihydroxy-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one |
| 122C412 | 21-Chloro-9-fluoro-11beta,17-dihydroxy-16beta-methylpregna-1,4-diene-3,20-dione | 21-Chloro-9-fluoro-11beta,17-dihydroxy-16beta-methylpregna-1,4-diene-3,20-dione (Clobetasol); |
| 25122-41-2 | AC-1775 | ADN79D536H |
| AKOS025401451 | API0024630 | CAS-25122-41-2 |
| CHEBI:205919 | CHEMBL1201362 | Clobecort Amex (TN) |
| Clobetasol (INN) | Clobetasol Propionate EP Impurity G | Clobetasol [INN:BAN] |
| Clobetasol, >=95.0% (HPLC), pharmaceutical impurity standard | Clobetasolum | Clobetasolum [INN-Latin] |
| D07715 | DB11750 | DSSTox_CID_28881 |
| DSSTox_GSID_48955 | DSSTox_RID_83149 | DTXSID2048955 |
| EINECS 246-633-8 | FT-0665105 | HSDB 7994 |
| J-015820 | M194 | NCGC00164580-01 |
| Pregna-1,4-diene-3,20-dione, 21-chloro-9-fluoro-11,17-dihydroxy-16-methyl-, (11beta,16beta)- | Q4224007 | SCHEMBL3996 |
| Tox21_113384 | UNII-ADN79D536H | ZINC26892643 |
| clobetasol |
| DrugBank Name | clobetasol |
| DrugBank | DB11750 |
| CAS Number | 25122-41-2 |
| PubChem Compound | 5311051 |
| KEGG Drug | D07715 |
| ChEBI | 205919 |