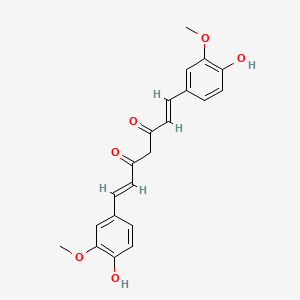
D0859 | curcumin
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| MEMBRANE POTENTIAL | 1.31±1.03 | human | qHTS-HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 2.51 | human | HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 11.60±9.87 | rat | hepatocytes | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | > 200 µM | 30 mins | mouse | liver mitochondria | Rh123 fluorescence (excitation 485 nm, emission 535 nm) are recorded using a fluorescence multi-well plate reader (mCICCP (20 µM) treatments was considered as the 100% baseline for ΔΨm loss) | decrease | EC20 | 36 |
| RESPIRATION | > 200 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | decrease | EC20 | 36 |
| RESPIRATION | > 200 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | decrease | EC20 | 36 |
| SWELLING | > 200 µM | 30 mins | mouse | liver mitochondria | swelling assay: Absorbance at 545 nm using a fluorescence multi-well plate reader (CaCl2 (50 µM) was considered as the 100% baseline for the swelling ) | increase | EC20 | 36 |
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| NADH:ubiquinone reductase | > 200 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | inhibit | EC20 | 36 |
| Succinate dehydrogenase | > 200 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | inhibit | EC20 | 36 |
| Cytochrome c | > 200 µM | 30 mins | mouse | liver mitochondria | Cytochrome c release was evaluated using ELISA kit ( 20 µg/ml Alamethicin was used as 100% baseline) | release | EC20 | 36 |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Danger |
Aggregated GHS information provided by 54 companies from 1 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H304 (100%): May be fatal if swallowed and enters airways [Danger Aspiration hazard] H317 (100%): May cause an allergic skin reaction [Warning Sensitization, Skin] H412 (100%): Harmful to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P261, P272, P273, P280, P301+P310, P302+P352, P321, P331, P333+P313, P363, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
 |
Warning |
Aggregated GHS information provided by 285 companies from 10 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. Reported as not meeting GHS hazard criteria by 12 of 285 companies. For more detailed information, please visit ECHA C&L website Of the 9 notification(s) provided by 273 of 285 companies with hazard statement code(s): H315 (99.63%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (99.27%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 540mg/kg (540mg/kg) | Yakugaku Zasshi. Journal of Pharmacy. Vol. 81, Pg. 659, 1961. | |
| rat | LD50 | oral | 1650mg/kg (1650mg/kg) | Toxicology and Applied Pharmacology. Vol. 1, Pg. 240, 1959. | |
| rat | LD50 | intraperitoneal | 634mg/kg (634mg/kg) | Nippon Yakurigaku Zasshi. Japanese Journal of Pharmacology. Vol. 62, Pg. 11, 1966. | |
| dog | LDLo | intravenous | 260mg/kg (260mg/kg) | National Technical Information Service. Vol. PB282-666, | |
| hamster | LD50 | oral | 1690mg/kg (1690mg/kg) | Pharmazie. Vol. 8, Pg. 572, 1953. | |
| mouse | LD50 | oral | 866mg/kg (866mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 28, Pg. 1644, 1978. | |
| mouse | LC50 | inhalation | 33900mg/m3 (33900mg/m3) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 34(10), Pg. 36, 1969. | |
| guinea pig | LD50 | oral | 1870mg/kg (1870mg/kg) | lungs, thorax, or respiration: respiratory depression | Toxicology and Applied Pharmacology. Vol. 2, Pg. 23, 1960. |
| rabbit | LD50 | subcutaneous | 1gm/kg (1000mg/kg) | behavioral: convulsions or effect on seizure threshold | Arzneimittel-Forschung. Drug Research. Vol. 21, Pg. 719, 1971. |
| mouse | LD50 | subcutaneous | 1625mg/kg (1625mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 8, Pg. 25, 1958. | |
| man | LDLo | unreported | 74mg/kg (74mg/kg) | "Poisoning; Toxicology, Symptoms, Treatments," 2nd ed., Arena, J.M., Springfield, IL, C.C. Thomas, 1970Vol. 2, Pg. 73, 1970. | |
| rabbit | LD50 | oral | 2500mg/kg (2500mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 21(9), Pg. 53, 1977. | |
| ((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) | (1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-3-methoxy-phenyl)-hepta-1,4,6-trien-3-one | (1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione |
| (1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione # | (1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione | (1E,6E)-1,7-Bis-(4-hydroxy-3-methoxy-phenyl)-hepta-1,6-diene-3,5-dione |
| (1E,6E)-1,7-bis(3-methoxy-4-oxidanyl-phenyl)hepta-1,6-diene-3,5-dione | (1E,6E)-1,7-bis(4-hydroxy-3-methoxy-phenyl)hepta-1,6-diene-3,5-dione | (1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione. |
| (1E,6E)-1,7-bis[4-hydroxy-3-(methyloxy)phenyl]hepta-1,6-diene-3,5-dione | (1Z,6E)-1,7-Bis-(4-hydroxy-3-methoxy-phenyl)-hepta-1,6-diene-3,5-dione | (E,E)-1,7-bis(4-Hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione |
| 1,3-Di(3-methoxy-4-hydroxystyryl)propanedial | 1,5-Di(vanillyliden)acetylaceton | 1,5-Divanillyliden-2,4-pentandion |
| 1,5-dione, 1,7-bis(4-hydroxy-3-methoxyphenyl)- | 1,6-Heptadiene-3,5-dione, 1,7-bis(4-hydroxy-3-methoxyphenyl)- | 1,6-Heptadiene-3,5-dione, 1,7-bis(4-hydroxy-3-methoxyphenyl)-, (1E,6E)- |
| 1,6-Heptadiene-3,5-dione, 1,7-bis(4-hydroxy-3-methoxyphenyl)-, (E,E)- | 1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione | 1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione |
| 1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, (E,E)- | 1,7-Bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione | 1,7-Bis-(4-hydroxy-3-methoxy-phenyl)-hepta-1,6-diene-3,5-dione |
| 1,7-DI(4-HYDROXY-3-METHOXYPHENYL)HEPTA-1,6-DIENE-3 | 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadien-3,5-dione | 1,7-bis(4-hydroxy-3-methoxyphenyl)1,6-heptadiene-3,5-dione |
| 1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione | 1,9-Bis(4-hydroxy-3-methoxyphenyl)-2,7-nonadiene-4,6-dione | 1790-EP2305629A1 |
| 1790-EP2308861A1 | 2,7-Nonadiene-4,6-dione, 1,9-bis(4-hydroxy-3-methoxyphenyl)- | 4-08-00-03697 (Beilstein Handbook Reference) |
| 458-37-7 | 458C377 | 5-Hydroxy-1,7-bis-(4-hydroxy-3-methoxy-phenyl)-hepta-1,4,6-trien-3-one |
| 5-hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,4,6-heptatrien-3-one | 8024-37-1 | 91884-86-5 |
| 98% curcurmin) | AC-24238 | AKOS001305497 |
| AS-72202 | BBL027711 | BB_NC-01422 |
| BCP04695 | BCP0726000035 | BCP9000557 |
| BDBM29532 | BDBM50067040 | BDBM50140172 |
| BG0601 | BIC8500 | BIDD:ER0479 |
| BRD-K07572174-001-02-2 | BRD-K07572174-001-19-6 | BRD-K07572174-001-22-0 |
| BRN 2306965 | C-230 | C.I. 75300 |
| C.I. Natural Yellow 3 | C21H20O6 | CAS-458-37-7 |
| CC0179 | CCG-36020 | CCG-36107 |
| CCRIS 3257 | CCRIS 5804 | CHEBI:3962 |
| CHEMBL140 | CI 75300 | CI Natural Yellow 3 |
| CS-1490 | CU-01000001305-2 | CURCUMIN (SEE ALSO TUMERIC, OLEORESIN (10105-J)) |
| Cucurmin | Curcuma | Curcuma |
| Curcuma longa l. oil | Curcuma longa l. oleoresin | Curcuma longa l. root oil |
| Curcuma longa l. root oleoresin | Curcuma longa oils | Curcuma oil |
| Curcuma oil (Curcuma longa) | Curcumin | Curcumin (Synthetic), 98% |
| Curcumin (mixture of curcumin, demethoxycurcumin, and bisdemethoxycurcumin), 98+% | Curcumin I | Curcumin solution, ~0.1 % (w/v) (in ethanol with 2M HCl (99:1 v/v)), for TLC derivatization; |
| Curcumin, >=94% (curcuminoid content), >=80% (Curcumin) | Curcumin, United States Pharmacopeia (USP) Reference Standard | Curcumin, analytical standard |
| Curcumin, from Curcuma longa (Turmeric), powder | Curcumin, matrix substance for MALDI-MS, >=99.5% (HPLC) | Curcumin, primary pharmaceutical reference standard |
| Curcumin,(S) | Curcumine | Curcurmin |
| DB-002681 | DB11672 | DSSTox_CID_1421 |
| DSSTox_GSID_31077 | DSSTox_RID_78861 | DTXSID8031077 |
| Diferaloylmethane | Diferuloylmethane | E 100 |
| E 100 (Dye) | EINECS 207-280-5 | FEMA No. 3085 |
| FEMA No. 3086 | GP8291 | GTPL7000 |
| Gelbwurz | Golden seal | HMS2233K04 |
| HMS3649K06 | HSDB 4334 | HY-N0005 |
| Haidr | Halad | Haldar |
| Haldar, Souchet | Halud | Hydrastis |
| IT942ZTH98 | Indian saffron | Indian turmeric |
| J10108 | K00009 | Kacha haldi |
| Kurkumin | Kurkumin [Czech] | LS-125 |
| LS-2189 | M212 | MFCD00008365 |
| MLS000069631 | MLS001148449 | Merita earth |
| N1839 | NCGC00017159-04 | NCGC00017159-05 |
| NCGC00017159-06 | NCGC00017159-07 | NCGC00017159-09 |
| NCGC00017159-10 | NCGC00017159-11 | NCGC00017159-12 |
| NCGC00023332-03 | NCGC00023332-04 | NCGC00023332-05 |
| NCGC00258668-01 | NCI-C61325 | NSC 32982 |
| NSC 687842 | NSC-32982 | NSC32982 |
| NSC687842 | Natural yellow 3 | OR24598 |
| Oil of turmeric | Oils, curcuma | Opera_ID_1627 |
| Orange Root | PREVENTION 4 (CURCUMIN) (SEE ALSO TUMERIC, OLEORESIN (10105-J)) | Q312266 |
| RTR-032605 | SB17248 | SBB006495 |
| SC-17381 | SCHEMBL13521974 | SCHEMBL8440 |
| SCHEMBL8441 | SMR000058237 | SR-01000000149 |
| SR-01000000149-2 | SR-01000000149-5 | ST055629 |
| STL371943 | Safran d'Inde | Souchet |
| TR-032605 | TURMERIC, OLEORESIN (CURCUMIN) (SEE ALSO CURCURMIN (458-37-7)) | Terra Merita |
| Tox21_110803 | Tox21_111505 | Tox21_201116 |
| Tu rmeric root oil | Tumeric oleoresin | Tumeric yellow |
| Turmeric | Turmeric | Turmeric (>98% curcurmin) |
| Turmeric extract | Turmeric extract (Curcuma longa L.) | Turmeric oil |
| Turmeric oil (Curcuma longa L.) | Turmeric oleoresin | Turmeric oleoresin (79%-85% curcumin) |
| Turmeric root oil | Turmeric root oleoresin | Turmeric yellow |
| Turmeric, oleoresin | UNII-IT942ZTH98 | VFLDPWHFBUODDF-FCXRPNKRSA-N |
| W-5038 | WLN: 1OR BQ E1U1V1V1U1R DQ CO1 | Yellow Ginger |
| Yellow Root | Yellow puccoon | Yo-Kin |
| ZINC899824 | ZX-AT003872 | Zlut prirodni 3 |
| Zlut prirodni 3 [Czech] | cMAP_000052 | cid_5281767 |
| cid_969516 | curcuma longa l. root oil CO2 extract | curcuma longa l. root oil hydrodistilled |
| curcumin | curouma | diferuloylmethan |
| kachs haldi | safra d'inde | trans,trans-Curcumin |
| turmeric root oil CO2 extract | turmeric root oil hydrodistilled |