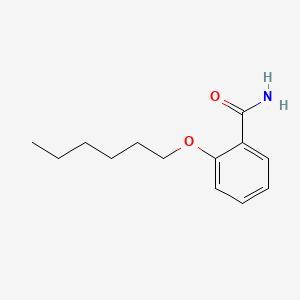
Compound
D0922 | exalamide
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| MEMBRANE POTENTIAL | 9.81±3.00 | human | qHTS-HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | human | HepG2 | MMP assay | Negative | IC50 | 163 | ||
| MEMBRANE POTENTIAL | 29.15±2.37 | rat | hepatocytes | MMP assay | decrease | IC50 | 163 | |
| 2-(Hexyloxy)benzamide | 2-Hexyloxybenzamide | 2-hexoxybenzamide |
| 2-n-Hexyloxybenzamide | 370E904 | 53370-90-4 |
| 7JEC65JCG2 | A829549 | AB00052353_02 |
| AKOS016014129 | BEN685 | BENZAMIDE, o-HEXYLOXY- |
| BRD-K12609457-001-02-3 | BRN 2645003 | Benzamide, 2-(hexyloxy)- |
| CAS-53370-90-4 | CCG-39743 | CHEBI:31585 |
| CHEMBL1405973 | CKSJXOVLXUMMFF-UHFFFAOYSA-N | CS-4872 |
| CTK8F9710 | D01585 | DB-052320 |
| DSSTox_CID_25899 | DSSTox_GSID_45899 | DSSTox_RID_81211 |
| DTXSID9045899 | DivK1c_000140 | EINECS 258-504-3 |
| Exalamida | Exalamida [INN-Spanish] | Exalamide (JAN/INN) |
| Exalamide [INN:JAN] | Exalamidum | Exalamidum [INN-Latin] |
| FT-0630660 | H.P. 216 | HMS1922C12 |
| HMS500G22 | HY-B1224 | Hyperan |
| IDI1_000140 | KBio1_000140 | KBio2_002209 |
| KBio2_004777 | KBio2_007345 | KBioSS_002209 |
| LS-26914 | NCGC00095049-01 | NCGC00095049-02 |
| NCGC00095049-04 | NINDS_000140 | NSC-758449 |
| NSC758449 | Pharmakon1600-01503403 | Q27114461; |
| SBI-0051822.P002 | SCHEMBL151112 | SPBio_000917 |
| SPECTRUM1503403 | SR-01000872750 | SR-01000872750-1 |
| Spectrum2_000869 | Spectrum5_001095 | Spectrum_001729 |
| Tox21 111403 | Tox21_111403 | Tox21_111403_1 |
| UNII-7JEC65JCG2 | VA10874 | ZINC1542919 |
| exalamide | o-(Hexyloxy)benzamide | o-Hexyloxybenzamide |
| ortho-hexyloxybenzamide |