Drug
D0986 | manidipine
C
C09BB12 Delapril and manidipine
[C09BB] ACE inhibitors and calcium channel blockers
[C09B] ACE INHIBITORS, COMBINATIONS
[C09] AGENTS ACTING ON THE RENIN-ANGIOTENSIN SYSTEM
[C] Cardiovascular system
C08CA11 Manidipine
[C08CA] Dihydropyridine derivatives
[C08C] SELECTIVE CALCIUM CHANNEL BLOCKERS WITH MAINLY VASCULAR EFFECTS
[C08] CALCIUM CHANNEL BLOCKERS
[C] Cardiovascular system
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| MEMBRANE POTENTIAL | 12.15±6.82 | human | qHTS-HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 27.48 | human | HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 20.91±5.06 | rat | hepatocytes | MMP assay | decrease | IC50 | 163 | |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Danger |
The GHS information provided by 1 company from 1 notification to the ECHA C&L Inventory. H301 (100%): Toxic if swallowed [Danger Acute toxicity, oral] |
P264, P270, P301+P310, P321, P330, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 171mg/kg (171mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 17(Suppl, | |
| rat | LD50 | subcutaneous | 199mg/kg (199mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 17(Suppl, | |
| mouse | LD50 | subcutaneous | 340mg/kg (340mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 17(Suppl, | |
| rat | LD50 | intraperitoneal | 49mg/kg (49mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 17(Suppl, | |
| rat | LD50 | oral | 156mg/kg (156mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 17(Suppl, | |
| mouse | LD50 | intraperitoneal | 62mg/kg (62mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 17(Suppl, | |
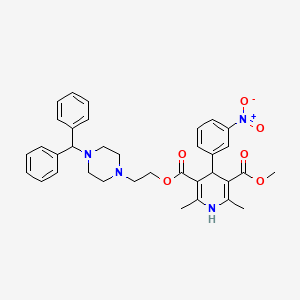
| 092M684 | 120092-68-4 | 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylic acid O5-[2-[4-(diphenylmethyl)-1-piperazinyl]ethyl] ester O3-methyl ester |
| 2-(4-Diphenylmethyl-1-piperazinyl)ethyl methyl-1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylate | 2-[4-(diphenylmethyl)piperazin-1-yl]ethyl methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate | 3,5-Pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 2-(4-(diphenylmethyl)-1-piperazinyl)ethyl methyl ester |
| 3,5-Pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 3-[2-[4-(diphenylmethyl)-1-piperazinyl]ethyl] 5-methyl ester | 3,5-Pyridinedicarboxylicacid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-,3-[2-[4-(diphenylmethyl)-1-piperazinyl]ethyl] 5-methyl ester, hydrochloride(1:2) | 3-(2-(4-Benzhydrylpiperazin-1-yl)ethyl) 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine- |
| 3-(2-(4-Benzhydrylpiperazin-1-yl)ethyl) 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate | 3-{2-[4-(diphenylmethyl)piperazin-1-yl]ethyl} 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate | 4CH-007438 |
| 5-O-[2-(4-benzhydrylpiperazin-1-yl)ethyl] 3-O-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate | 89226-50-6 | 89226-75-5 |
| A804429 | AB0012678 | AB01274741-01 |
| AB01274741-02 | AB01274741-03 | AB01274741_04 |
| AB01274741_05 | AB2000589 | ACMC-20conv |
| AK-72917 | AKOS003589070 | AKOS016842350 |
| ANEBWFXPVPTEET-UHFFFAOYSA-N | Artedil | Artedil (TN) |
| BCP21717 | BCP9000893 | BCP9000919 |
| BDBM50227969 | BR-72917 | BRD-A90695733-001-01-7 |
| C-23346 | C35H38N4O6 | CCG-213069 |
| CHEBI:135849 | CHEMBL312176 | CS-2523 |
| CTK5G2647 | CV 4093 | CV 4093;CV-4093;CV4093;Franidipine;89226-50-6 |
| D08155 | DB-041526 | DB09238 |
| DS-1275 | DTXSID2043745 | FT-0631098 |
| FT-0670936 | Franidipine | HMS2089K12 |
| HMS3264H11 | HMS3656C06 | HY-B0419 |
| Iperten | KS-00000GIQ | LS-130875 |
| MCULE-7098869865 | MLS004774156 | MLS006011791 |
| Manidipine (INN) | Manidipine (Manyper) | Manidipine 6300 |
| Manidipine 6300 [INN] | Manidipine HCl | Manidipine [INN] |
| Manidipine pound>>CV-4093 pound>>Franidipine pound>>( inverted exclamation markA)-Manidipine | Manidipine(Manyper) | NCGC00167493-01 |
| NCGC00167493-02 | NCGC00167493-03 | NCGC00167493-04 |
| O5-[2-[4-(diphenylmethyl)piperazin-1-yl]ethyl] O3-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate; | Q921133 | S-6064 |
| SC-25949 | SCHEMBL49368 | SMR003500793 |
| SR-05000002134 | SR-05000002134-1 | ST24028270 |
| STK635322 | SW219347-1 | TR-038262 |
| VA11233 | VP13803 | Z5288 |
| manidipine | s2481 |
| DrugBank Name | manidipine |
| DrugBank | DB09238 |
| CAS Number | 120092-68-4, 126451-47-6, 133082-19-6, 89226-50-6, 89226-75-5, 94849-74-8 |
| PubChem Compound | 4008 |
| KEGG Drug | D08155 |
| ChEBI | 135849 |