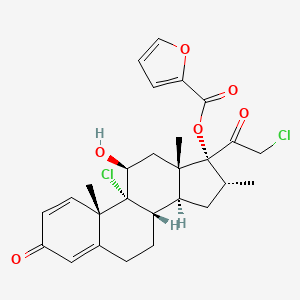
D1005 | mometasone furoate
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| MEMBRANE POTENTIAL | 11.80±0.80 | human | qHTS-HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 8.3 | human | HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 13.96±1.14 | rat | hepatocytes | MMP assay | decrease | IC50 | 163 | |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Danger |
Aggregated GHS information provided by 122 companies from 12 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H360 (94.26%): May damage fertility or the unborn child [Danger Reproductive toxicity] H370 (43.44%): Causes damage to organs [Danger Specific target organ toxicity, single exposure] H372 (63.93%): Causes damage to organs through prolonged or repeated exposure [Danger Specific target organ toxicity, repeated exposure] H373 (31.15%): Causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure] H410 (31.15%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P260, P264, P270, P273, P281, P307+P311, P308+P313, P314, P321, P391, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | subcutaneous | 300mg/kg (300mg/kg) | lungs, thorax, or respiration: respiratory depression | Kiso to Rinsho. Clinical Report. Vol. 24, Pg. 4203, 1990. |
| mouse | LDLo | subcutaneous | 1gm/kg (1000mg/kg) | Kiso to Rinsho. Clinical Report. Vol. 24, Pg. 4203, 1990. | |
| (11beta,16alpha)-9,21-Dichloro-17-[(2-furanylcarbonyl)oxy]-11-hydroxy-16-methylpregna-1,4-diene-3,20-dione | (11beta,16alpha)-9,21-dichloro-11-hydroxy-16-methyl-3,20-dioxopregna-1,4-dien-17-yl furan-2-carboxylate | (8S,9R,10S,11S,13S,14S,16R,17R)-9-chloro-17-(2-chloroacetyl)-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl furan-2-carboxylate |
| (9beta,10alpha,11alpha,14beta,16alpha,17alpha)-9,21-dichloro-11-hydroxy-16-methyl-3,20-dioxopregna-1,4-dien-17-yl furan-2-carboxylate | 04201GDN4R | 11?,16?)-9,21-Dichloro-11-hydroxy-16-methyl-3,20-dioxopregna-1,4-dien-17-yl 2-furoate |
| 2-furancarboxylic acid [(8S,9R,10S,11S,13S,14S,16R,17R)-9-chloro-17-(2-chloro-1-oxoethyl)-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] ester | 83919-23-7 | 9,21-Dichloro-11beta,17-dihydroxy-16alpha-methylpregna-1,4-diene-3,20-dione 17-(2-furoate) |
| 9,21-dichloro-11beta-hydroxy-16alpha-methyl-3,20-dioxopregna-1,4-dien-17-yl furan-2-carboxylate | 919M237 | A840685 |
| AB2000407 | AC-941 | AKOS015994732 |
| Asmanex | Asmanex (TN) | Asmanex HFA |
| Asmanex Twisthaler | BDBM50148733 | BPBio1_000424 |
| BRD-K60640630-001-03-7 | BRD-K60640630-001-11-0 | BRN 4340538 |
| BSPBio_000384 | C07817 | C27H30Cl2O6 |
| CAS-83919-23-7 | CCG-220572 | CHEBI:47564 |
| CHEMBL1161 | CS-2389 | Certified Reference Material |
| D00690 | DB14512 | DSSTox_CID_3333 |
| DSSTox_GSID_23333 | DSSTox_RID_76981 | DTXSID4023333 |
| Danitin | Ecural | Elocon |
| Elocon (TN) | Elocone | Elomet |
| Flumeta | HMS1569D06 | HMS2096D06 |
| HMS2235I14 | HMS3713D06 | HY-13693 |
| KS-00000SPL | KS-1275 | LAS 41002 |
| M2354 | MLS002153879 | MOF |
| MOMETASONE FUROATE MONOHYDRATE | Mometasone 17-(2-furoate) | Mometasone Furoate Impurity G |
| Mometasone Furoate, Pharmaceutical Secondary Standard | Mometasone fuorate | Mometasone furoate (JAN/USP) |
| Mometasone furoate [USAN:USP:JAN] | Mometasone furoate anhydrous | Mometasone furoate, >=98% (HPLC) |
| Mometasone furoate, European Pharmacopoeia (EP) Reference Standard | Mometasone furoate, United States Pharmacopeia (USP) Reference Standard | Monovo |
| NCGC00016950-01 | NCGC00179578-01 | NCGC00179578-03 |
| NCGC00179578-04 | NSC-746171 | NSC746171 |
| Nasonex | Nosorex | Ovixan |
| Pregna-1,20-dione, 9,21-dichloro-17-[(2-furanylcarbonyl)oxy]-11-hydroxy-16-methyl-, (11.beta.,16.alpha.)-; | Pregna-1,4-diene-3,20-dione, 9,21-dichloro-17-((2-furanylcarbonyl)oxy)-11-hydroxy-16-methyl-, (11beta,16alpha)- | Prestwick0_000572 |
| Prestwick1_000572 | Prestwick2_000572 | Prestwick3_000572 |
| Prestwick_924 | Q-101380 | Q1044248 |
| Rimelon | SCHEMBL4568 | SMR001233233 |
| SPBio_002603 | SR-01000841209 | SR-01000841209-2 |
| ST24048112 | Sch 32088 | Sch-32088 |
| Sch32088 | Sinuva | Tox21_110705 |
| Tox21_110705_1 | UNII-04201GDN4R | WOFMFGQZHJDGCX-ZULDAHANSA-N |
| ZINC3938677 | [(8S,9R,10S,11S,13S,14S,16R,17R)-9-chloranyl-17-(2-chloranylethanoyl)-10,13,16-trimethyl-11-oxidanyl-3-oxidanylidene-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] furan-2-carboxylate | [(8S,9R,10S,11S,13S,14S,16R,17R)-9-chloro-17-(2-chloroacetyl)-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] furan-2-carboxylate |
| mometasone 17-furoate | mometasone furancarboxylate | mometasone furoate |
| mometasone-furoate | s1987 |