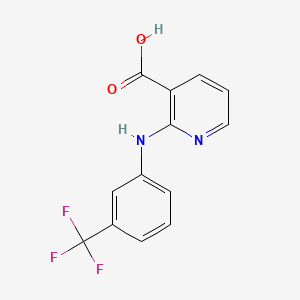
D1014 | niflumic acid
M
M02AA17 Niflumic acid
[M02AA] Antiinflammatory preparations, non-steroids for topical use
[M02A] TOPICAL PRODUCTS FOR JOINT AND MUSCULAR PAIN
[M02] TOPICAL PRODUCTS FOR JOINT AND MUSCULAR PAIN
[M] Musculoskeletal system
M01AX02 Niflumic acid
[M01AX] Other antiinflammatory and antirheumatic agents, non-steroids
[M01A] ANTIINFLAMMATORY AND ANTIRHEUMATIC PRODUCTS, NON-STEROIDS
[M01] ANTIINFLAMMATORY AND ANTIRHEUMATIC PRODUCTS
[M] Musculoskeletal system
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| MEMBRANE POTENTIAL | 13.02±2.17 | human | qHTS-HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 15.45 | human | HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 6.71±1.14 | rat | hepatocytes | MMP assay | decrease | IC50 | 163 | |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Warning |
Aggregated GHS information provided by 45 companies from 6 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H302 (93.33%): Harmful if swallowed [Warning Acute toxicity, oral] H312 (93.33%): Harmful in contact with skin [Warning Acute toxicity, dermal] H315 (95.56%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (95.56%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H332 (93.33%): Harmful if inhaled [Warning Acute toxicity, inhalation] H335 (93.33%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure Respiratory tract irritation] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P403+P233, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 350mg/kg (350mg/kg) | United States Patent Document. Vol. #4122202, | |
| rat | LD50 | intraperitoneal | 100mg/kg (100mg/kg) | United States Patent Document. Vol. #3466373, | |
| guinea pig | LD50 | intravenous | 174mg/kg (174mg/kg) | United States Patent Document. Vol. #3466373, | |
| mouse | LD50 | intravenous | 152mg/kg (152mg/kg) | Therapie. Vol. 22, Pg. 157, 1967. | |
| mouse | LD50 | intraperitoneal | 196mg/kg (196mg/kg) | Nippon Yakurigaku Zasshi. Japanese Journal of Pharmacology. Vol. 68, Pg. 683, 1972. | |
| rat | LD50 | oral | 250mg/kg (250mg/kg) | behavioral: analgesia | Journal of Medicinal Chemistry. Vol. 16, Pg. 780, 1973. |
| 1td7 | 2(3'-trifluormethylanilino)-nicotinic acid | 2-((3-(Trifluoromethyl)phenyl)amino)nicotinic acid |
| 2-(3-(Trifluoromethyl)-phenyl)aminonicotinic acid | 2-(3-(Trifluoromethyl)anilino)nicotinic acid | 2-(3-(Trifluoromethyl)phenylamino)nicotinic acid |
| 2-(3-Trifluoromethyl-phenylamino)-nicotinic acid | 2-(3-Trifluoromethylanilino)nicotinic Acid | 2-(3-[Trifluoromethyl]anilino)nicotinic acid |
| 2-(3-trifluoromethylphenoxy)nicotinamide | 2-(alpha,alpha,alpha-Trifluoro-m-toluidino)nicotinic acid | 2-(|A,|A,|A-Trifluoro-m-toluidino)nicotinic acid; |
| 2-[(3-TRIFLUOROMETHYL)PHENYL]AMINO-3-PYRIDINE-CARBOXYLIC ACID | 2-[(3-Trifluoromethyl)phenyl]amino]-3-pyridinecarboxylic Acid | 2-[(3-Trifluoromethylphenyl)amino]nicotinic Acid |
| 2-[3-(Trifluoromethyl)anilino]-3-pyridinecarboxylic acid | 2-[3-(Trifluoromethyl)anilino]nicotinic acid | 2-[3-(Trifluoromethyl)anilino]nicotinic acid |
| 2-[3-(trifluoromethyl)anilino]pyridine-3-carboxylic acid | 2-[[3-(trifluoromethyl)phenyl]amino]pyridine-3-carboxylic acid | 2-{[3-(TRIFLUOROMETHYL)PHENYL]AMINO}NICOTINIC ACID |
| 2-{[3-(trifluoromethyl)phenyl]amino}pyridine-3-carboxylic acid | 3-Pyridinecarboxylic acid, 2-((3-(trifluoromethyl)phenyl)amino)- | 3-Pyridinecarboxylic acid, 2-[[3-(trifluoromethyl)phenyl]amino]- |
| 394N007 | 4394-00-7 | 4U5MP5IUD8 |
| 5-22-13-00598 (Beilstein Handbook Reference) | AB00052255 | AB00052255-17 |
| AB00052255_18 | AB00052255_19 | AB0012229 |
| AB1004097 | AC-2652 | ACMC-209jvx |
| AK-72979 | AKOS000519590 | AM20070143 |
| ANW-30043 | Acide niflumique | Acide niflumique [French] |
| Acide niflumique [INN-French] | Acido niflumico | Acido niflumico [INN-Spanish] |
| Acido niflumico [Italian] | Acidum niflumicum | Acidum niflumicum [INN-Latin] |
| Actol | Actol, analgesic | Aza-2 dimethyl-2',3' (tetrazolyl-5)-6 diphenylamino |
| Aza-2 dimethyl-2',3' (tetrazolyl-5)-6 diphenylamino [French] | BBL003619 | BDBM85507 |
| BPBio1_000078 | BR-72979 | BRD-K98763141-001-04-3 |
| BRD-K98763141-001-06-8 | BRD-K98763141-001-17-5 | BRN 0489360 |
| BSPBio_000070 | BSPBio_001393 | BSPBio_003069 |
| Bio1_000114 | Bio1_000603 | Bio1_001092 |
| Bio2_000113 | Bio2_000593 | C13698 |
| CAS-4394-00-7 | CAS_4394-00-7 | CBiol_001828 |
| CCG-40157 | CCRIS 5740 | CHEBI:34888 |
| CHEMBL63323 | CS-2614 | CTK4I7886 |
| D08275 | DB04552 | DL-457 |
| DSSTox_CID_3368 | DSSTox_GSID_23368 | DSSTox_RID_76996 |
| DTXSID1023368 | DivK1c_000277 | Donalgin |
| EINECS 224-516-2 | EU-0100845 | FT-0603659 |
| Forenol | GS-3202 | GTPL2439 |
| HMS1361F15 | HMS1568D12 | HMS1791F15 |
| HMS1921D12 | HMS1989F15 | HMS2090D19 |
| HMS2095D12 | HMS2234F11 | HMS3262J11 |
| HMS3374H01 | HMS3402F15 | HMS3649A08 |
| HMS3656P14 | HMS3712D12 | HMS500N19 |
| HY-B0493 | IDI1_000277 | IDI1_033863 |
| JZFPYUNJRRFVQU-UHFFFAOYSA-N | KBio1_000277 | KBio2_000113 |
| KBio2_001833 | KBio2_002681 | KBio2_004401 |
| KBio2_005249 | KBio2_006969 | KBio3_000225 |
| KBio3_000226 | KBio3_002569 | KBioGR_000113 |
| KBioGR_000505 | KBioSS_000113 | KBioSS_001833 |
| KS-00000CB5 | KSC587Q8N | LP00845 |
| LS-96639 | Landruma | Lopac-N-0630 |
| Lopac0_000845 | MCULE-3027710274 | MFCD00010569 |
| MLS000069713 | MLS001076327 | N 0630 |
| NCGC00015724-01 | NCGC00015724-02 | NCGC00015724-03 |
| NCGC00015724-04 | NCGC00015724-05 | NCGC00015724-06 |
| NCGC00015724-07 | NCGC00015724-08 | NCGC00015724-09 |
| NCGC00015724-10 | NCGC00015724-11 | NCGC00015724-12 |
| NCGC00015724-13 | NCGC00015724-14 | NCGC00015724-17 |
| NCGC00023636-03 | NCGC00023636-04 | NCGC00023636-05 |
| NCGC00023636-06 | NCGC00023636-07 | NCGC00023636-08 |
| NCGC00023636-09 | NCGC00261530-01 | NFL |
| NINDS_000277 | NSC-758196 | NSC758196 |
| NSC_4488 | Nicotinic acid, 2-(.alpha.,.alpha.,.alpha.-trifluoro-m-toluidino)- | Nicotinic acid, 2-(alpha,alpha,alpha-trifluoro-m-toluidino)- |
| Niflamol | Niflugel (TN) | Niflumate |
| Niflumic acid (Hit 16) | Niflumic acid (INN) | Niflumic acid [INN:BAN:DCF] |
| Niflumic acid [INN:DCF] | Niflumic acid, European Pharmacopoeia (EP) Reference Standard | NiflumicAcid |
| Nifluminic acid | Nifluril | Opera_ID_1746 |
| Pharmakon1600-01502015 | Prestwick0_000255 | Prestwick1_000255 |
| Prestwick2_000255 | Prestwick3_000255 | Prestwick_890 |
| PubChem21796 | Q304285 | RTR-017015 |
| S00109 | SBB001146 | SBI-0050821.P003 |
| SC 1332 | SCHEMBL24706 | SMR000058199 |
| SPBio_000928 | SPBio_002289 | SPECTRUM1502015 |
| SR-01000000231 | SR-01000000231-11 | SR-01000000231-2 |
| SR-01000000231-5 | SR-01000000231-6 | ST013871 |
| ST24027408 | STK803109 | SW197011-3 |
| Spectrum2_000794 | Spectrum3_001485 | Spectrum4_000043 |
| Spectrum5_001216 | Spectrum_001353 | T7852 |
| Tox21_110206 | Tox21_110206_1 | Tox21_500845 |
| UNII-4U5MP5IUD8 | UP 83 | UP-83 |
| W-106215 | ZINC125031 | ni-flumic acid |
| niflumic acid | niflumic-acid | s3018 |