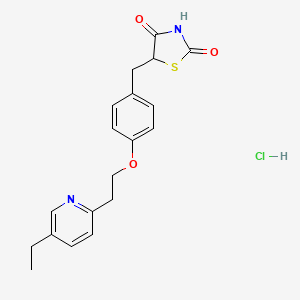
D1058 | pioglitazone hydrochloride
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| MEMBRANE POTENTIAL | human | qHTS-HepG2 | MMP assay | Negative | IC50 | 163 | ||
| MEMBRANE POTENTIAL | human | HepG2 | MMP assay | Negative | IC50 | 163 | ||
| MEMBRANE POTENTIAL | 8.90±9.20 | rat | hepatocytes | MMP assay | decrease | IC50 | 163 | |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Warning |
Aggregated GHS information provided by 55 companies from 14 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. Reported as not meeting GHS hazard criteria by 1 of 55 companies. For more detailed information, please visit ECHA C&L website Of the 13 notification(s) provided by 54 of 55 companies with hazard statement code(s): H302 (12.96%): Harmful if swallowed [Warning Acute toxicity, oral] H319 (81.48%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P264, P270, P280, P301+P312, P305+P351+P338, P330, P337+P313, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| (+-)-5-(p-(2-(5-Ethyl-2-pyridyl)ethoxy)benzyl)-2,4-thiazolidinedione monohydrochloride | 025P468 | 112529-15-4 |
| 2,4-Thiazolidinedione, 5-((4-(2-(5-ethyl-2-pyridinyl)ethoxy)phenyl)methyl)-, monohydrochloride, (+-)- | 2,4-Thiazolidinedione, 5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-, monohydrochloride | 2,4-thiazolidinedione,5-((4-(2-(5-ehtyl-2-pyridinyl)ethoxy)phenyl)methyl)-monohydrochloride; |
| 5-((4-(2-(5-ethyl-2-pyridinyl)ethoxy)phenyl)methyl)-2,4-thiazolidinedione, hcl | 5-(4-(2-(5-Ethylpyridin-2-yl)ethoxy)benzyl)thiazolidine-2,4-dione hydrochloride | 5-(4-[2-(5-Ethyl-2-pyridyl)ethoxy]benzyl)thiazolidine-2,4-dione hydrochloride |
| 5-(4-[2-(5-ethyl-pyridin-2-yl)-ethoxy]-benzyl)-thiazolidine-2,4-dione hcl | 5-(4-[2-(5-ethyl-pyridin-2-yl)-ethoxy]-benzyl)-thiazolidine-2,4-dione hydrochloride | 5-({4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl}methyl)-1,3-thiazolidine-2,4-dione hydrochloride |
| 5-[4-[2-(5-Ethyl-2-pyridyl)ethoxy]benzyl]-2,4-thiazolidinedione Hydrochloride | 5-[4-[2-(5-ethyl-2-pyridyl)ethoxy]benzyl]thiazolidine-2,4-dione hydrochloride | 5-[4-[2-(5-ethyl-pyridin-2-yl)-ethoxy]-benzyl]-thiazolidine-2,4-dione hydrochloride |
| 5-[[4-[2-(5-Ethyl-2-pyridinyl)-ethoxy]phenyl]methyl]-2,4-thiazolidinedione hydrochloride | 5-[[4-[2-(5-Ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-thiazolidinedione monohydrochloride | 5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-thiazolidinedione, hydrochloride |
| 5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]thiazolidine-2,4-dione hydrochloride | 5-[[4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione hydrochloride | 5-[[4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione;hydrochloride |
| 5-{4-[2-(5-Ethyl-pyridin-2-yl)-ethoxy]-benzyl}-thiazolidine-2,4-dione HCl | 5-{4-[2-(5-Ethyl-pyridin-2-yl)-ethoxy]-benzyl}-thiazolidine-2,4-dione hydrochloride | 5-{4-[2-(5-ethylpyridin-2-yl)ethoxy]benzyl}-1,3-thiazolidine-2,4-dione hydrochloride |
| A-2403 | A802593 | AA-10090 |
| AB0013914 | AB1009260 | AC-1037 |
| ACT04238 | AD 4833 | AK-50391 |
| AKOS015844016 | AM20061770 | ANW-42027 |
| Actos (TN) | BCP0726000151 | BCP22942 |
| BR-50391 | C19H20N2O3S.HCl | C19H21ClN2O3S |
| CAS-112529-15-4 | CCG-100931 | CCG-39097 |
| CHEBI:8229 | CHEMBL1715 | CP0139 |
| CPD000469167 | CS-2235 | CTK8B3228 |
| Certified Reference Material | D00945 | DSSTox_CID_24203 |
| DSSTox_GSID_44203 | DSSTox_RID_80116 | DTXSID3044203 |
| FT-0082357 | FT-0601607 | GHUUBYQTCDQWRA-UHFFFAOYSA-N |
| Glustin | HMS1922L05 | HY-14601 |
| I868 | J10107 | KS-00000IO7 |
| KS-1186 | LS-151328 | LS-172192 |
| MCULE-1259842295 | MFCD04975446 | MLS001306462 |
| MLS001401386 | NC00181 | NCGC00095131-01 |
| NCGC00095131-02 | NCGC00163128-08 | NCGC00254492-01 |
| P1901 | Pioditazone hydrochloride | Pioglitazone (hydrochloride) |
| Pioglitazone HCl | Pioglitazone Hydrochloride ,(S) | Pioglitazone for system suitability, European Pharmacopoeia (EP) Reference Standard |
| Pioglitazone hydrochloride (Actos) | Pioglitazone hydrochloride (JP17/USP) | Pioglitazone hydrochloride [USAN:USP] |
| Pioglitazone hydrochloride [USAN] | Pioglitazone hydrochloride, >=98% (HPLC) | Pioglitazone hydrochloride, European Pharmacopoeia (EP) Reference Standard |
| Pioglitazone hydrochloride, Pharmaceutical Secondary Standard | Pioglitazone hydrochloride, United States Pharmacopeia (USP) Reference Standard | Piomed |
| Q-201584 | Q27281642 | RTR-001112 |
| SAM001246600 | SB17324 | SC-19064 |
| SCHEMBL21843 | SMR000469167 | SPECTRUM1504401 |
| ST2411660 | STR-001 | SW197561-4 |
| TR-001112 | Tox21_111440 | Tox21_111440_1 |
| Tox21_300584 | U 72107A | U-72107A |
| U-72107E | [(+-)-5-[[4-[4-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-] monohydrochloride | [5-[[4-[2-(5-Ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-] thiazolidinedione hydrochloride |
| pioglitazone hydrochloride | pioglitazone, hydrochloride | pioglitazone-hydrochloride-actos |
| s2046 |