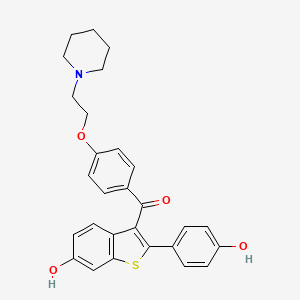
D1081 | raloxifene
G
G03XC01 Raloxifene
[G03XC] Selective estrogen receptor modulators
[G03X] OTHER SEX HORMONES AND MODULATORS OF THE GENITAL SYSTEM
[G03] SEX HORMONES AND MODULATORS OF THE GENITAL SYSTEM
[G] Genitourinary system and reproductive hormones
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| MEMBRANE POTENTIAL | 5.05±3.99 | human | qHTS-HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 17.78 | human | HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 22.40±24.05 | rat | hepatocytes | MMP assay | decrease | IC50 | 163 | |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Warning |
The GHS information provided by 1 company from 1 notification to the ECHA C&L Inventory. H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (100%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H332 (100%): Harmful if inhaled [Warning Acute toxicity, inhalation] H335 (100%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure Respiratory tract irritation] |
P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| women | TDLo | oral | 36mg/kg/30D-I (36mg/kg) | Lancet. Vol. 352, Pg. 1524, 1998. | |
| (2-(4-Hydroxyphenyl)-6-hydroxybenzo(b)thien-3-yl)(4-(2-(1-piperidinyl)ethoxy)phenyl)methanone | (6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thiophen-3-yl)(4-(2-(piperidin-1-yl)ethoxy)phenyl)methanone | 15189-EP2270008A1 |
| 15189-EP2272825A2 | 15189-EP2280012A2 | 15189-EP2281815A1 |
| 15189-EP2289892A1 | 15189-EP2292615A1 | 15189-EP2292617A1 |
| 15189-EP2295426A1 | 15189-EP2295427A1 | 15189-EP2301928A1 |
| 15189-EP2301933A1 | 15189-EP2305640A2 | 15189-EP2305671A1 |
| 15189-EP2308855A1 | 15189-EP2311808A1 | 15189-EP2311825A1 |
| 15189-EP2311827A1 | 15189-EP2311829A1 | 15189-EP2311840A1 |
| 15189-EP2311842A2 | 15189-EP2314581A1 | 15189-EP2316832A1 |
| 15189-EP2316833A1 | 2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethoxy]phenyl}carbonyl)-1-benzothiophen-6-ol | 2-(4-hydroxyphenyl)-3-{4-[2-(piperidin-1-yl)ethoxy]benzoyl}-1-benzothiophen-6-ol |
| 449R901 | 6-Hydroxy-2-(4-Hydroxyphenyl)-3-[4(2-Piperidinoethoxy)benzoyl]benzo[b]thiophene | 6-hydroxy-2-(4-hydoxyphenyl)-3-[4-(2-piperidinoethoxy)benzoyl]benzo[b]thiophene |
| 6-hydroxy-2-(4-hydoxyphenyl)-3[-4-(2-piperidinoethoxy)benzoyl]benzo[b]thiophene | 6-hydroxy-2-(4-hydroxyphenyl)-3-[4-(2-piperidinoethoxy)benzoyl]benzo[b]-thiophene | 6-hydroxy-2-(4-hydroxyphenyl)-3-[4-(2-piperidinoethoxy)benzoyl]benzo[b]thiophene |
| 6-hydroxy-2-(4-hydroxyphenyl)-3-[4-(2piperidinoethoxy)-benzoyl]benzo[b]thiophene | 6-hydroxy-2-(4-hydroxyphenyl)-3[-4-(2-piperidinoethoxy)benzoyl]benzo [b]thiophene | 6-hydroxy-2-(4-hydroxyphenyl)-3[-4-(2-piperidinoethoxy)benzoyl]benzo[b]thiophene |
| 6-hydroxy-2-(4-hydroxyphenyl)-3[-4-(2piperidinoethoxy)benzoyl]benzo[b]thiophene | 6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thien-3-yl 4-[2-(1-piperidinyl)ethoxy]phenyl methanone; | 6-hydroxy-2-(4-hyroxyphenyl)-3-[4-(2-piperidinoethoxy)benzoyl]benzo[b]thiophene |
| 84449-90-1 | AB0073011 | AC-8399 |
| AKOS015896267 | API0003075 | AS-35086 |
| BCP09772 | BDBM19441 | BIDD:ER0216 |
| BIDD:GT0795 | BPBio1_000995 | BRD-K63828191-003-11-5 |
| BSPBio_000903 | C-22734 | C07228 |
| C28H27NO4S | CAS-82640-04-8 | CAS-84449-90-1 |
| CC-34210 | CCG-205128 | CCRIS 7129 |
| CHEBI:8772 | CHEMBL81 | D08465 |
| DB00481 | DSSTox_CID_3550 | DSSTox_GSID_23550 |
| DSSTox_RID_77076 | DTXSID3023550 | Eviden (TN) |
| FT-0630926 | GTPL2820 | GZUITABIAKMVPG-UHFFFAOYSA-N |
| HMS2089F06 | HMS3742O11 | HSDB 7460 |
| J22.982B | KBio2_002361 | KBio2_004929 |
| KBio2_007497 | KBio3_002840 | KBioGR_002361 |
| KBioSS_002364 | Keoxifene | LS-177821 |
| LY 139481 | LY-139481 | LY-156758 |
| LY139481 | Lopac-R-1402 | Lopac0_001051 |
| MCULE-4598311006 | MRF-0000684 | Methanone, (6-hydroxy-2-(4-hydroxyphenyl)benzo(b)thien-3-yl)(4-(2-(1-piperidinyl)ethoxy)phenyl)- |
| Methanone, [6-Hydroxy-2-(4-hydroxyphenyl)benzo[b]thien-3-yl][4-[2-(1-piperidinyl)ethoxy]phenyl]- | NCGC00015889-01 | NCGC00015889-02 |
| NCGC00015889-04 | NCGC00015889-05 | NCGC00015889-06 |
| NCGC00015889-07 | NCGC00015889-08 | NCGC00015889-10 |
| NCGC00092353-02 | NCGC00092353-04 | NCGC00260151-01 |
| NSC-747974 | NSC747974 | Optruma |
| Pharoxifene | Prestwick0_000862 | Prestwick1_000862 |
| Prestwick2_000862 | Prestwick3_000862 | Q425223 |
| RAL | Raloxifene (INN) | Raloxifene [INN:BAN] |
| Raloxifene, 6 | Raloxifeno | Raloxifeno [Spanish] |
| Raloxifenum | Raloxifenum [Latin] | Raxeto (TN) |
| SBI-0051021.P002 | SC-17311 | SCHEMBL6144 |
| SMP2_000095 | SPBio_002824 | Tox21_202603 |
| UNII-YX9162EO3I | VU0155042-3 | YX9162EO3I |
| ZINC538275 | [2-(4-Hydroxyphenyl)-6-hydroxybenzo[b]thien-3-yl][4-(2-(1-piperidinyl)ethoxy)phenyl]methanone | [2-(4-hydroxyphenyl)-6-hydroxybenzo[b]thien-3-yl][4-[2-(1-piperidinyl)ethoxy]phenyl]methanone |
| [6-Hydroxy-2-(4-hydroxy-phenyl)-benzo[b]thiophen-3-yl]-[4-(2-piperidin-1-yl-ethoxy)-phenyl]-methanone | [6-hydroxy-2-(4-hydroxyphenyl)-1-benzothien-3-yl][4-(2-piperidin-1-ylethoxy)phenyl]methanone | [6-hydroxy-2-(4-hydroxyphenyl)-1-benzothien-3-yl]{4-[(2-piperidin-1-ylethyl)oxy]phenyl}methanone |
| [6-hydroxy-2-(4-hydroxyphenyl)-1-benzothiophen-3-yl]-[4-(2-piperidin-1-ylethoxy)phenyl]methanone | [6-hydroxy-2-(4-hydroxyphenyl)-1-benzothiophen-3-yl][4-(2-piperidin-1-ylethoxy)phenyl]methanone | [6-hydroxy-2-(4-hydroxyphenyl)benzo-[b]thien-3-yl][4-[2-(1-piperidinyl)ethoxy]phenyl]methanone |
| [6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thien-3-yl]-[4-[2-(1-piperidinyl)ethoxy]phenyl]methanone | [6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thien-3-yl]-[4-[2-(1piperidinyl)ethoxy]phenyl]methanone | [6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thien-3-yl][4-[2-(1-piperidinyl)ethoxy]phenyl]methanone |
| [6-hydroxy-2-(4-hydroxyphenyl)benzothiophen-3-yl]-[4-[2-(1-piperidyl)ethoxy]phenyl]methanone | cMAP_000032 | cid_11071264 |
| raloxifene | s5781 |