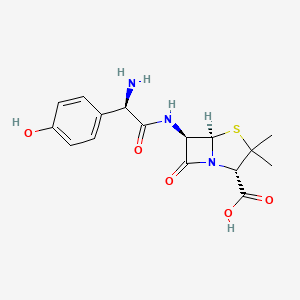
D1295 | Amoxicillin
J
A
J01CR50 Combinations of penicillins
[J01CR] Combinations of penicillins, incl. beta-lactamase inhibitors
[J01C] BETA-LACTAM ANTIBACTERIALS, PENICILLINS
[J01] ANTIBACTERIALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
J01CA04 Amoxicillin
[J01CA] Penicillins with extended spectrum
[J01C] BETA-LACTAM ANTIBACTERIALS, PENICILLINS
[J01] ANTIBACTERIALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
A02BD15 Vonoprazan, amoxicillin and metronidazole
[A02BD] Combinations for eradication of Helicobacter pylori
[A02B] DRUGS FOR PEPTIC ULCER AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
[A02] DRUGS FOR ACID RELATED DISORDERS
[A] Alimentary tract and metabolism
A02BD14 Vonoprazan, amoxicillin and clarithromycin
[A02BD] Combinations for eradication of Helicobacter pylori
[A02B] DRUGS FOR PEPTIC ULCER AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
[A02] DRUGS FOR ACID RELATED DISORDERS
[A] Alimentary tract and metabolism
A02BD13 Rabeprazole, amoxicillin and metronidazole
[A02BD] Combinations for eradication of Helicobacter pylori
[A02B] DRUGS FOR PEPTIC ULCER AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
[A02] DRUGS FOR ACID RELATED DISORDERS
[A] Alimentary tract and metabolism
A02BD12 Rabeprazole, amoxicillin and clarithromycin
[A02BD] Combinations for eradication of Helicobacter pylori
[A02B] DRUGS FOR PEPTIC ULCER AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
[A02] DRUGS FOR ACID RELATED DISORDERS
[A] Alimentary tract and metabolism
A02BD11 Pantoprazole, amoxicillin, clarithromycin and metronidazole
[A02BD] Combinations for eradication of Helicobacter pylori
[A02B] DRUGS FOR PEPTIC ULCER AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
[A02] DRUGS FOR ACID RELATED DISORDERS
[A] Alimentary tract and metabolism
A02BD10 Lansoprazole, amoxicillin and levofloxacin
[A02BD] Combinations for eradication of Helicobacter pylori
[A02B] DRUGS FOR PEPTIC ULCER AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
[A02] DRUGS FOR ACID RELATED DISORDERS
[A] Alimentary tract and metabolism
A02BD07 Lansoprazole, amoxicillin and clarithromycin
[A02BD] Combinations for eradication of Helicobacter pylori
[A02B] DRUGS FOR PEPTIC ULCER AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
[A02] DRUGS FOR ACID RELATED DISORDERS
[A] Alimentary tract and metabolism
A02BD06 Esomeprazole, amoxicillin and clarithromycin
[A02BD] Combinations for eradication of Helicobacter pylori
[A02B] DRUGS FOR PEPTIC ULCER AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
[A02] DRUGS FOR ACID RELATED DISORDERS
[A] Alimentary tract and metabolism
A02BD05 Omeprazole, amoxicillin and clarithromycin
[A02BD] Combinations for eradication of Helicobacter pylori
[A02B] DRUGS FOR PEPTIC ULCER AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
[A02] DRUGS FOR ACID RELATED DISORDERS
[A] Alimentary tract and metabolism
A02BD04 Pantoprazole, amoxicillin and clarithromycin
[A02BD] Combinations for eradication of Helicobacter pylori
[A02B] DRUGS FOR PEPTIC ULCER AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
[A02] DRUGS FOR ACID RELATED DISORDERS
[A] Alimentary tract and metabolism
A02BD03 Lansoprazole, amoxicillin and metronidazole
[A02BD] Combinations for eradication of Helicobacter pylori
[A02B] DRUGS FOR PEPTIC ULCER AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
[A02] DRUGS FOR ACID RELATED DISORDERS
[A] Alimentary tract and metabolism
A02BD01 Omeprazole, amoxicillin and metronidazole
[A02BD] Combinations for eradication of Helicobacter pylori
[A02B] DRUGS FOR PEPTIC ULCER AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
[A02] DRUGS FOR ACID RELATED DISORDERS
[A] Alimentary tract and metabolism
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| MEMBRANE POTENTIAL | > 400 µM | 30 mins | mouse | liver mitochondria | Rh123 fluorescence (excitation 485 nm, emission 535 nm) are recorded using a fluorescence multi-well plate reader (mCICCP (20 µM) treatments was considered as the 100% baseline for ΔΨm loss) | decrease | EC20 | 36 |
| RESPIRATION | 90.8 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | increase | EC20 | 36 |
| RESPIRATION | 188.8 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | increase | EC20 | 36 |
| SWELLING | > 400 µM | 30 mins | mouse | liver mitochondria | swelling assay: Absorbance at 545 nm using a fluorescence multi-well plate reader (CaCl2 (50 µM) was considered as the 100% baseline for the swelling ) | increase | EC20 | 36 |
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| Cytochrome c | > 400 µM | 30 mins | mouse | liver mitochondria | Cytochrome c release was evaluated using ELISA kit ( 20 µg/ml Alamethicin was used as 100% baseline) | release | EC20 | 36 |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Danger |
Aggregated GHS information provided by 56 companies from 4 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H317 (100%): May cause an allergic skin reaction [Warning Sensitization, Skin] H334 (98.21%): May cause allergy or asthma symptoms or breathing difficulties if inhaled [Danger Sensitization, respiratory] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P261, P272, P280, P285, P302+P352, P304+P341, P321, P333+P313, P342+P311, P363, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
  |
Danger |
H317: May cause an allergic skin reaction [Warning Sensitization, Skin] H334: May cause allergy or asthma symptoms or breathing difficulties if inhaled [Danger Sensitization, respiratory] |
P261, P272, P280, P285, P302+P352, P304+P341, P321, P333+P313, P342+P311, P363, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | oral | > 15gm/kg (15000mg/kg) | Drugs in Japan Vol. 6, Pg. 38, 1982. | |
| mouse | LD50 | intraperitoneal | 3590mg/kg (3590mg/kg) | Drugs in Japan Vol. -, Pg. 59, 1990. | |
| man | TDLo | oral | 40mg/kg/4D (40mg/kg) | Lancet. Vol. 2, Pg. 707, 1978. | |
| rat | LD50 | intraperitoneal | 2870mg/kg (2870mg/kg) | Drugs in Japan Vol. -, Pg. 59, 1990. | |
| child | TDLo | oral | 682mg/kg (682mg/kg) | Clinical Pediatrics Vol. 32, Pg. 735, 1993. | |
| rat | LD50 | subcutaneous | > 8gm/kg (8000mg/kg) | Drugs in Japan Vol. -, Pg. 59, 1990. | |
| mouse | LD50 | intracrebral | > 500mg/kg (500mg/kg) | brain and coverings: recordings from specific areas of cns | Chemotherapy Vol. 26, Pg. 196, 1980. |
| mouse | LD50 | subcutaneous | > 20mg/kg (20mg/kg) | Drugs in Japan Vol. -, Pg. 59, 1990. | |
| mouse | LD50 | oral | > 25gm/kg (25000mg/kg) | Drugs in Japan Vol. 6, Pg. 38, 1982. | |
| man | TDLo | oral | 7143ug/kg (7.143mg/kg) | skin and appendages (skin): "dermatitis, other: after systemic exposure" | Allergy. Vol. 52(Suppl, |
| child | TDLo | oral | 300mg/kg (300mg/kg) | Clinical Pediatrics Vol. 32, Pg. 735, 1993. | |