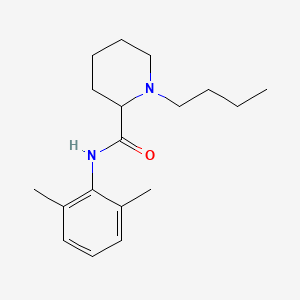
D0016 | Bupivacaine
N
N01BB51 Bupivacaine, combinations
[N01BB] Amides
[N01B] ANESTHETICS, LOCAL
[N01] ANESTHETICS
[N] Nervous system
N01BB01 Bupivacaine
[N01BB] Amides
[N01B] ANESTHETICS, LOCAL
[N01] ANESTHETICS
[N] Nervous system
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| DEPOLARIZATION | decrease | 61 | ||||||
| OPENING OF PERMEABILITY TRANSITION PORE (PTP) | decrease | 61 | ||||||
| UNCOUPLING | rat | isolated liver mitochondria | measurements of mitochondrial respiration; RST inhibition assay, RST uncoupling assay; IC 50ratio of glucose/galactose assay | Negative | 53 | |||
| ELECTROPHORETIC UNCOUPLING | 278 | |||||||
| MEMBRANE POTENTIAL | 258.2 µM | 30 mins | mouse | liver mitochondria | Rh123 fluorescence (excitation 485 nm, emission 535 nm) are recorded using a fluorescence multi-well plate reader (mCICCP (20 µM) treatments was considered as the 100% baseline for ΔΨm loss) | decrease | EC20 | 36 |
| RESPIRATION | decrease | 61 | ||||||
| RESPIRATION | 60.6 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | decrease | EC20 | 36 |
| RESPIRATION | > 800 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | decrease | EC20 | 36 |
| ELECTRON TRANSPORT CHAIN | rat | isolated liver mitochondria | measurements of mitochondrial respiration; RST inhibition assay, RST uncoupling assay; IC 50ratio of glucose/galactose assay | Negative | 53 | |||
| SWELLING | > 800 µM | 30 mins | mouse | liver mitochondria | swelling assay: Absorbance at 545 nm using a fluorescence multi-well plate reader (CaCl2 (50 µM) was considered as the 100% baseline for the swelling ) | increase | EC20 | 36 |
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| NADH:ubiquinone reductase | 60.6 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | inhibit | EC20 | 36 |
| Succinate dehydrogenase | > 800 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | inhibit | EC20 | 36 |
| Cytochrome c | > 800 µM | 30 mins | mouse | liver mitochondria | Cytochrome c release was evaluated using ELISA kit ( 20 µg/ml Alamethicin was used as 100% baseline) | release | EC20 | 36 |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Danger |
The GHS information provided by 1 company from 1 notification to the ECHA C&L Inventory. H300 (100%): Fatal if swallowed [Danger Acute toxicity, oral] H310 (100%): Fatal in contact with skin [Danger Acute toxicity, dermal] H330 (100%): Fatal if inhaled [Danger Acute toxicity, inhalation] |
P260, P262, P264, P270, P271, P280, P284, P301+P310, P302+P350, P304+P340, P310, P320, P321, P322, P330, P361, P363, P403+P233, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
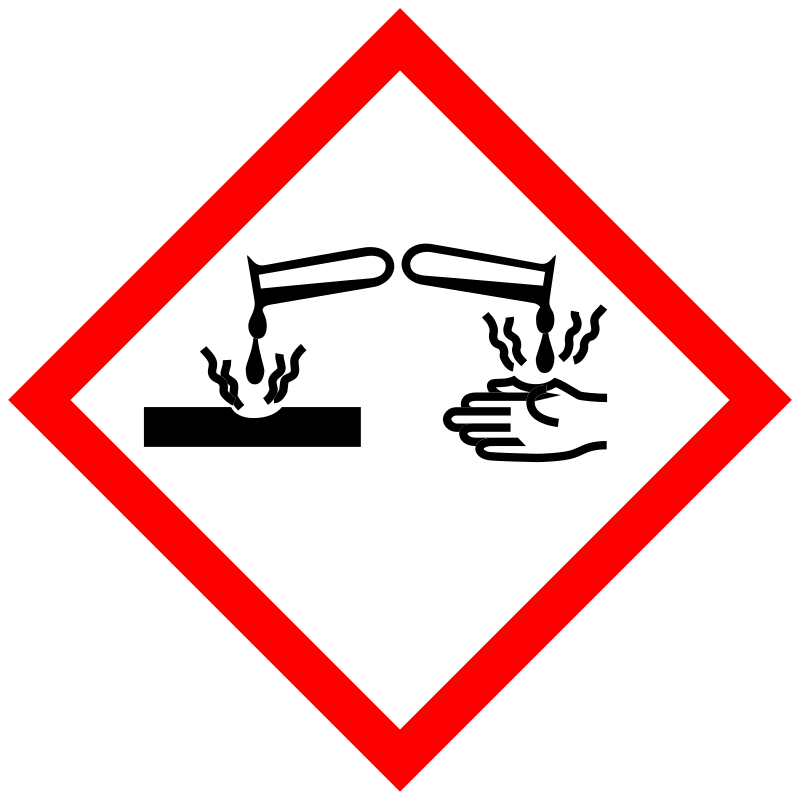   |
Danger |
The GHS information provided by 1 company from 1 notification to the ECHA C&L Inventory. H300 (100%): Fatal if swallowed [Danger Acute toxicity, oral] H310 (100%): Fatal in contact with skin [Danger Acute toxicity, dermal] H315 (100%): Causes skin irritation [Warning Skin corrosion/irritation] H318 (100%): Causes serious eye damage [Danger Serious eye damage/eye irritation] H330 (100%): Fatal if inhaled [Danger Acute toxicity, inhalation] |
P260, P262, P264, P270, P271, P280, P284, P301+P310, P302+P350, P302+P352, P304+P340, P305+P351+P338, P310, P320, P321, P322, P330, P332+P313, P361, P362, P363, P403+P233, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| rabbit | LD50 | intratracheal | 12500ug/kg (12.5mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 26, Pg. 78, 1976. | |
| rabbit | LD50 | oral | 18mg/kg (18mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 200, Pg. 359, 1972. | |
| rabbit | LD50 | intratracheal | 11mg/kg (11mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 200, Pg. 359, 1972. | |
| mouse | LD50 | intravenous | 7100ug/kg (7.1mg/kg) | United States Patent Document. Vol. #3632766, | |
| rabbit | LD50 | parenteral | 64mg/kg (64mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 26, Pg. 78, 1976. | |
| rat | LD50 | subcutaneous | 52mg/kg (52mg/kg) | Acta Pharmacologica et Toxicologica. Vol. 31, Pg. 273, 1972. | |
| mouse | LD50 | subcutaneous | 100mg/kg (100mg/kg) | Acta Pharmacologica et Toxicologica. Vol. 31, Pg. 273, 1972. | |
| rabbit | LD50 | intravenous | 1620ug/kg (1.62mg/kg) | Anesthesia and Analgesia Vol. 64, Pg. 209, 1985. | |
| mouse | LD50 | intravenous | 9600ug/kg (9.6mg/kg) | Acta Pharmacologica et Toxicologica. Vol. 31, Pg. 273, 1972. | |
| guinea pig | LD50 | intraperitoneal | 50mg/kg (50mg/kg) | Drugs in Japan Vol. 6, Pg. 680, 1982. | |
| rabbit | LD50 | intratracheal | 14mg/kg (14mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 26, Pg. 78, 1976. | |
| rat | LD50 | intravenous | 7200ug/kg (7.2mg/kg) | Acta Pharmacologica et Toxicologica. Vol. 31, Pg. 273, 1972. | |
| rabbit | LDLo | intravenous | 9700ug/kg (9.7mg/kg) | behavioral: convulsions or effect on seizure threshold | Acta Pharmacologica et Toxicologica. Vol. 31, Pg. 273, 1972. |
| rat | LD50 | parenteral | 30mg/kg (30mg/kg) | Anesthesiology. Vol. 89, Pg. 1199, 1998. | |
| rat | LD50 | intravenous | 6mg/kg (6mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 200, Pg. 359, 1972. | |
| mouse | LD50 | intramuscular | 22753ug/kg (22.753mg/kg) | Drug Development Research. Vol. 21, Pg. 277, 1990. | |
| mouse | LD50 | subcutaneous | 59mg/kg (59mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 200, Pg. 359, 1972. | |
| rat | LD50 | subcutaneous | 43mg/kg (43mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 200, Pg. 359, 1972. | |
| mouse | LD50 | intravenous | 6100ug/kg (6.1mg/kg) | Anesthesia Progress. Vol. 36, Pg. 79, 1989. | |
| rabbit | LD50 | parenteral | 48mg/kg (48mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 200, Pg. 359, 1972. | |
| rat | LD50 | subcutaneous | 47mg/kg (47mg/kg) | United States Patent Document. Vol. #3632766, | |
| rabbit | LD50 | parenteral | > 120mg/kg (120mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 26, Pg. 78, 1976. | |
| domestic animals - goat/sheep | LDLo | intravenous | 150mg/kg (150mg/kg) | Anesthesia and Analgesia Vol. 86, Pg. 797, 1998. | |
| mouse | LD50 | subcutaneous | 35mg/kg (35mg/kg) | United States Patent Document. Vol. #3632766, | |
| guinea pig | LD50 | intraperitoneal | 36600ug/kg (36.6mg/kg) | United States Patent Document. Vol. #3632766, | |
| human | TDLo | intravenous | 4300ug/kg (4.3mg/kg) | Acta Anaesthesiologica Scandinavica. Vol. 21, Pg. 521, 1977. | |
| rabbit | LD50 | intravenous | 3400ug/kg (3.4mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 200, Pg. 359, 1972. | |
| rat | LD50 | intravenous | 5600ug/kg (5.6mg/kg) | Acta Pharmacologica et Toxicologica. Vol. 31, Pg. 273, 1972. | |
| mouse | LD50 | intraperitoneal | 58700ug/kg (58.7mg/kg) | Anesthesia and Analgesia Vol. 60, Pg. 385, 1981. | |
| (+-)-Bupivacaine | (+/-)-Bupivacaine | (.+/-.)-1-Butyl-2',6'-pipecoloxylidide |
| (.+/-.)-Bupivacaine | (1)-1-Butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide | (RS)-bupivacaine |
| (plusmn)-1-butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide | 1-Butyl-2',6'-pipecoloxylidide | 1-Butyl-N-(2,6-dimethylphenyl)-2-piperidinecarboxamide |
| 1-Butyl-N-(2,6-dimethylphenyl)-2-piperidinecarboximidic acid | 1-butyl-N-(2,6-dimethylphenyl)-piperidine-2-carboxamide | 1-butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide |
| 14252-80-3 (HCl) | 18010-40-7 (HCl) | 180B929 |
| 2',6'-Pipecoloxylidide, 1-butyl- | 2-Piperidinecarboxamide, 1-butyl-N-(2,6-dimethylphenyl)- | 2-Piperidinecarboxamide, 1-butyl-N-(2,6-dimethylphenyl)-, (+-)- |
| 2-Piperidinecarboxamide, 1-butyl-N-(2,6-dimethylphenyl)-, (.+/-.)- | 2180-92-9 | 38396-39-3 |
| 4CH-013994 | 73360-54-0 (HCl.H2O) | AB00053674 |
| AB00053674-08 | AB00053674-09 | AB00053674_10 |
| AB00053674_11 | AC-2096 | AH 250 |
| AK-90074 | AKOS001637202 | AKOS016842516 |
| ANW-45984 | API0007792 | AX8041457 |
| Anekain | BCP12242 | BCP21825 |
| BDBM50350790 | BPBio1_000298 | BRD-A01636364-003-05-2 |
| BRD-A01636364-003-08-6 | BS-5224 | BSPBio_000270 |
| BSPBio_002607 | Bloqueina | Bucaine (TN) |
| Bupivacaina | Bupivacaina [INN-Spanish] | Bupivacaine (USAN/INN) |
| Bupivacaine Carbonate | Bupivacaine HCL KIT | Bupivacaine Monohydrochloride, Monohydrate |
| Bupivacaine [INN:BAN] | Bupivacaine [USAN:INN:BAN] | Bupivacaine liposome injectable suspension |
| Bupivacaine,hydrochloride | Bupivacainum | Bupivacainum [INN-Latin] |
| C07529 | C18H28N2O | CHEBI:3215 |
| CHEBI:77431 | CHEMBL1098 | CS-W023182 |
| CTK8B4736 | Carbostesin | D07552 |
| DB-119016 | DB00297 | DL-Bupivacaine |
| DTXSID2022703 | DUR-843 | DivK1c_000758 |
| EINECS 218-553-3 | EINECS 253-911-2 | Epitope ID:122662 |
| Exparel | Exparel (TN) | FT-0623286 |
| FT-0699781 | GTPL2397 | HMS2090F12 |
| HMS3428P06 | HSDB 7790 | IDI1_000758 |
| J10217 | KBio1_000758 | KBio2_002004 |
| KBio2_004572 | KBio2_007140 | KBio3_001827 |
| KBioGR_001516 | KBioSS_002004 | KS-00000ZRC |
| L000695 | LAC-43 | LAC-43 (Salt/Mix) |
| LEBVLXFERQHONN-UHFFFAOYSA-N | LS-109841 | LS-2222 |
| Levobupivacaine free base | M-9606 | MCULE-2286784276 |
| MFCD00941462 | Marcaine | Marcaine hydrochloride (Salt/Mix) |
| Marcaine spinal (Salt/Mix) | N-(2,6-dimethylphenyl)(1-butyl(2-piperidyl))carboxamide | NCGC00178579-01 |
| NCGC00178579-02 | NINDS_000758 | Prestwick0_000305 |
| Prestwick1_000305 | Prestwick2_000305 | Prestwick3_000305 |
| Q-100271 | Q422806 | R962 |
| SBI-0051846.P002 | SC-26838 | SCHEMBL25438 |
| SKY 0402 | SKY-0402 | SKY0402 |
| SPBio_001558 | SPBio_002489 | ST065773 |
| ST2415271 | STL484283 | Sensorcaine |
| Spectrum2_001589 | Spectrum3_000974 | Spectrum4_001098 |
| Spectrum5_001483 | Spectrum_001524 | TC-134729 |
| Win 11318 | bupivacaine | bupivacaine base |
| cBupivacaine | dl-1-Butyl-2',6'-pipecoloxylidide | dl-1-Butyl-2,6-pipecoloxylidide |
| racemic bupivacaine |
| DrugBank Name | Bupivacaine |
| DrugBank | DB00297 |
| CAS Number | 119427-31-5, 14252-80-3, 2180-92-9, 27262-47-1, 38396-39-3, 4033-39-0, 474668-57-0 |
| PubChem Compound | 2474 |
| KEGG Compound ID | C07529 |
| KEGG Drug | D07552 |
| PubChem.Substance | 46506768 |
| ChEBI | 77431 |
| PharmGKB | PA135057240 |
| ChemSpider | 2380 |
| BindingDB | 50350790.0 |
| TTD | DAP001229 |
| Wikipedia | Bupivacaine |
| DPD | 2493 |