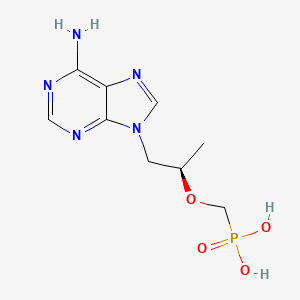
Drug
D0118 | Tenofovir
J
J05AF07 Tenofovir disoproxil
[J05AF] Nucleoside and nucleotide reverse transcriptase inhibitors
[J05A] DIRECT ACTING ANTIVIRALS
[J05] ANTIVIRALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| ELECTRON TRANSPORT CHAIN | decrease | 52 | ||||||
| MITOCHONDRIAL DNA METABOLIC PROCESS | 194 | |||||||
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| DNA polymerase gamma | 194 | |||||||
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Danger |
Aggregated GHS information provided by 2 companies from 1 notifications to the ECHA C&L Inventory. H318 (100%): Causes serious eye damage [Danger Serious eye damage/eye irritation] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P280, P305+P351+P338, and P310; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| (((1R)-2-(6-Amino-9H-purin-9-yl)-1-methylethoxy)methyl)phosphonic acid | (R)-(((1-(6-AMINO-9H-PURIN-9-YL)PROPAN-2-YL)OXY)METHYL)PHOSPHONIC ACID | (R)-(+)-9-[2-Phosphonylmethoxyethyl]adenine |
| (R)-(1-(6-amino-9H-purin-9-yl)propan-2-yloxy)methylphosphonic acid | (R)-9-(2-Phosphonomethoxypropyl)adenine | (R)-9-(2-Phosphonoylmethoxypropyl)adenine |
| (R)-9-(Phosphonomethoxypropyl)adenine | (R)-9-[2-(Phosphonomethoxy)propyl]adenine | (R)-9-[2-(phosphonomethoxy) propyl] adenine |
| (R)-PMPA | (R)-[[2-(6-Amino-9H-purin-9-yl)-1-methylethoxy]methyl]phosphonic acid | ({[(2R)-1-(6-amino-9H-purin-9-yl)propan-2-yl]oxy}methyl)phosphonic acid |
| 114611-EP2272516A2 | 114611-EP2298783A1 | 127T206 |
| 147127-20-6 | 24230-EP2298783A1 | 24230-EP2314590A1 |
| 9-PMPA | 9-[(R)-2-(phosphonomethoxy)propyl]adenine | AB0017664 |
| AB01274787-01 | AB01274787_02 | AB01274787_03 |
| ABP000917 | AC-760 | ACN-029240 |
| AK-33334 | AKOS015856701 | AKOS015894941 |
| AM20090678 | API0015388 | AX8018435 |
| BCPP000049 | BG0625 | BR-33334 |
| BRD-K15891719-001-02-8 | C17407 | CHEBI:63625 |
| CHEMBL483 | CP-558 | CS-1609 |
| CTK4C5270 | D,L-Tenofovir | DTXSID9040132 |
| EC 604-571-2 | FT-0601652 | GNA & Tenofovir |
| GS 1275 | GS 1278 | GS-1278 |
| HHA & Tenofovir | HMS3264H05 | HY-13910 |
| J90012 | KS-5021 | KSC525E7B |
| PMPA | PMPA gel | PMPA-(R) |
| Phosphonic acid, (((1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy)methyl)- | Phosphonic acid, ((2-(6-amino-9H-purin-9-yl)-1-methylethoxy)methyl)-, (R)- | Phosphonic acid, P-[[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]- |
| Phosphonic acid, [[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]- | Phosphonic acid, [[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]- & Galanthus nivalis agglutinin (GNA) | Phosphonic acid, [[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]- & Hippeastrum hybrid agglutinin( HHA) |
| PubChem9452 | Q-201787 | Q155954 |
| S-2389 | SC-01187 | SCHEMBL39724 |
| SGOIRFVFHAKUTI-ZCFIWIBFSA-N | SR-01000883934 | SR-01000883934-1 |
| ST24033314 | TFV gel | TR-035738 |
| Tenofovir | Tenofovir (Viread) | Tenofovir [USAN:INN:BAN] |
| Tenofovir gel | Tenofovir gel (GS-1278) | Tenofovir, >=98% (HPLC) |
| Tenofovir,TDF,PMPA/ | Truvada | UNII-W4HFE001U5 |
| Viread (prodrug for Tenofovir) | W4HFE001U5 | ZINC1543475 |
| [(1R)-2-(6-aminopurin-9-yl)-1-methyl-ethoxy]methylphosphonic acid | [2-(6-AMINO-9H-PURIN-9-YL)-1-METHYLETHOXY]METHYLPHOSPHONIC ACID | [[(1R)-2(6-Amino-9H-Purin-9-Yl)-1-Methylethoxy]Methyl]Phosphonic Acid |
| anh. tenofovir | anhydrous tenofovir | tenofovir (anh.) |
| tenofovir (anhydrous) | {[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl}phosphonic acid |
1. Dykens et al. (2007)