D0261 | Cyclosporin A
S
L
S01XA18 Ciclosporin
[S01XA] Other ophthalmologicals
[S01X] OTHER OPHTHALMOLOGICALS
[S01] OPHTHALMOLOGICALS
[S] Sensory organs
L04AD01 Ciclosporin
[L04AD] Calcineurin inhibitors
[L04A] IMMUNOSUPPRESSANTS
[L04] IMMUNOSUPPRESSANTS
[L] Antineoplastic and immunomodulating agents
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| TRANSMEMBRANE POTENTIAL | 40nM | rat | rat cortical neurons and isolated rat liver mitochondria | mitochondrial membrane potential was measured in tetramethylrhodamine methyl ester (TMRM)-loaded neurons using ImageXpress MicroXL, and in rhodamine-123 loaded isolated mitochondria using a fluorescent plate reader | Negative | 324 | ||
| DEPOLARIZATION | 1 μmol/L | 3 minutes | rat | liver mitochondria | Assessment of Mitochondrial Membrane Potential; coincubated with diclofenac | decrease | p < 0.05 | 6 |
| OPENING OF PERMEABILITY TRANSITION PORE (PTP) | inhibit | 248 | ||||||
| OPENING OF PERMEABILITY TRANSITION PORE (PTP) | 40nM | rat | liver mitochondria | Assess Ca2+-mediated PT pore formation by Calcium Retention Capacity Assay (Fluo-5N fluorescence was measured in the extramitochondrial solution following repeated additions of Ca2+) | inhibit | half-maximal inhibition | 324 | |
| OPENING OF PERMEABILITY TRANSITION PORE (PTP) | 5 µM | 1 hour | Human | HepG2 | High-content screening assay | Negative | MEC | 306 |
| MEMBRANE POTENTIAL | 5 µM | 1 hour | Human | HepG2 | High-content screening assay | Increase | MEC | 306 |
| MITOCHONDRIAL PARAMETERS | 1 μM | rat | rat cortical neurons and isolated rat liver mitochondria | oxygen consumption (Oroboros Oxygraph-2K) | Negative | 324 | ||
| ACCUMULATION OF CALCIUM | 1 μmol/L | 2 minutes | rat | liver mitochondria | Assessment of Mitochondrial Ca2+ Efflux; Incubated in the presence of Diclofenac(50 μmol/L); Energized with succinate | Negative | not mentioned | 6 |
| ATP SYNTHESIS | 1 μM | rat | rat cortical neurons and isolated rat liver mitochondria | ATP production ( Cell Titer Glo reagent) | Negative | 324 | ||
| MITOPHAGY | 5 μM | mouse embryonic fibroblasts (MEFs) and SH-SY5Y cells | Mitochondrial population levels were determined by flow cytometry using MitoTracker Deep Red (MTDR) dye | block | 252 | |||
| ROS PRODUCTION | 5 µM | 1 hour | Human | HepG2 | High-content screening assay | Increase | MEC | 306 |
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase F, mitochondrial | 5 μM | mouse embryonic fibroblasts (MEFs) and SH-SY5Y cells | Mitochondrial population levels were determined by flow cytometry using MitoTracker Deep Red (MTDR) dye | inhibitor | 252 | |||
| Peptidyl-prolyl cis-trans isomerase F, mitochondrial | 22.5 ± 4.3nM (CypA), 1.4 ± 1.6 nM( K133I CypD) | Fluorescence Polarization Assay to measure affinity of ligands to cyclophilins (fluorescein-PEG-CsA ligand as the probe) | bind | Ki | 324 | |||
| Reactive oxygen species | 5 µM | 1 hour | Human | HepG2 | High-content screening assay | increase | MEC | 306 |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Danger |
Aggregated GHS information provided by 403 companies from 15 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] H350 (99.26%): May cause cancer [Danger Carcinogenicity] H360 (97.52%): May damage fertility or the unborn child [Danger Reproductive toxicity] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P264, P270, P281, P301+P312, P308+P313, P330, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
  |
Danger |
H302: Harmful if swallowed [Warning Acute toxicity, oral] H350: May cause cancer [Danger Carcinogenicity] H360: May damage fertility or the unborn child [Danger Reproductive toxicity] H362: May cause harm to breast-fed children [Reproductive toxicity, effects on or via lactation] H370: Causes damage to organs [Danger Specific target organ toxicity, single exposure] H372: Causes damage to organs through prolonged or repeated exposure [Danger Specific target organ toxicity, repeated exposure] |
P201, P202, P260, P263, P264, P270, P281, P301+P312, P307+P311, P308+P313, P314, P321, P330, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | intraperitoneal | 147mg/kg (147mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 17, Pg. 365, 1986. | |
| human | TDLo | oral | 12mg/kg (12mg/kg) | Journal of Hypertension. Vol. 11, Pg. 1209, 1993. | |
| women | TDLo | oral | 1911mg/kg/91W (1911mg/kg) | British Journal of Rheumatology. Vol. 33, Pg. 90, 1994. | |
| man | TDLo | parenteral | 20mg/kg/5D-I (20mg/kg) | American Journal of Ophthalmology. Vol. 126, Pg. 607, 1998. | |
| man | TDLo | unreported | 30mg/kg/4D-I (30mg/kg) | kidney, ureter, and bladder: "changes in tubules (including acute renal failure, acute tubular necrosis)" | Annals of Internal Medicine. Vol. 107, Pg. 786, 1987. |
| rabbit | LD50 | oral | > 1gm/kg (1000mg/kg) | Archives of Toxicology. Vol. 53, Pg. 107, 1983. | |
| rat | LD50 | subcutaneous | 286mg/kg (286mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 17, Pg. 365, 1986. | |
| mouse | LD50 | intravenous | 96mg/kg (96mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 17, Pg. 365, 1986. | |
| mouse | LD50 | oral | 2329mg/kg (2329mg/kg) | Archives of Toxicology. Vol. 53, Pg. 107, 1983. | |
| man | TDLo | oral | 20mg/kg/2D-I (20mg/kg) | Lancet. Vol. 2, Pg. 1092, 1986. | |
| rat | LD50 | oral | 1480mg/kg (1480mg/kg) | Archives of Toxicology. Vol. 53, Pg. 107, 1983. | |
| rat | LD50 | intravenous | 24mg/kg (24mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 17, Pg. 365, 1986. | |
| women | TDLo | oral | 62500ug/kg/5D (62.5mg/kg) | kidney, ureter, and bladder: other changes | Lancet. Vol. 1, Pg. 1221, 1986. |
| man | TDLo | oral | 14286ug/kg (14.286mg/kg) | Annals of Pharmacotherpy. Vol. 34, Pg. 405, 2000. | |
| rabbit | LD50 | intravenous | 10mg/kg (10mg/kg) | Toxicologic Pathology. Vol. 14, Pg. 73, 1986. | |
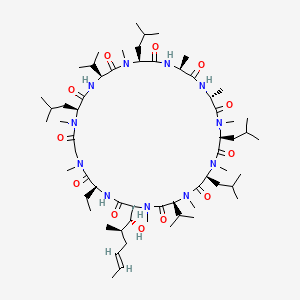
| (3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-Ethyl-33-[(1R,2R,4E)-1-hydroxy-2-methylhex-4-en-1-yl]-1,4,7,10,12,15,19,25,28-nonamethyl-6,9,18,24-tetrakis(2-methylpropyl)-3,21-di(propan-2-yl)-1,4,7,10,13,16,19,22,25,28,31-undecaazacyclotritriacontane-2,5,8,11,14,17,20,23,26,29,32-undecone | (3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33-((1R,2R,E)-1-hydroxy-2-methylhex-4-en-1-yl)-6,9,18,24-tetraisobutyl-3,21-diisopropyl-1,4,7,10,12,15,19,25,28-nonamethyl-1,4,7,10,13,16,1 | (3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33-((1R,2R,E)-1-hydroxy-2-methylhex-4-en-1-yl)-6,9,18,24-tetraisobutyl-3,21-diisopropyl-1,4,7,10,12,15,19,25,28-nonamethyl-1,4,7,10,13,16,19,22,25,28,31-undecaazacyclotritriacontan-2,5,8,11,14,17,20,23,26,29,32-undecaone |
| (3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33-[(1R,2R,4E)-1-hydroxy-2-methylhex-4-en-1-yl]-1,4,7,10,12,15,19,25,28-nonamethyl-6,9,18,24-tetrakis(2-methylpropyl)-3,21-bis(propan-2-yl)-1,4,7,10,13,16,19,22,25,28,31-undecaazacyclotritriacontan-2,5,8,11,14,17,20,23,26,29,32-undecone | (3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33-[(1R,2R,4E)-1-hydroxy-2-methylhex-4-en-1-yl]-6,9,18,24-tetraisobutyl-3,21-diisopropyl-1,4,7,10,12,15,19,25,28-nonamethyl-1,4,7,10,13,16,19,22,25,28,31-undecaazacyclotritriacontane-2,5,8,11,14,17,20,23,2 | (3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33-[(1R,2R,4E)-1-hydroxy-2-methylhex-4-en-1-yl]-6,9,18,24-tetraisobutyl-3,21-diisopropyl-1,4,7,10,12,15,19,25,28-nonamethyl-1,4,7,10,13,16,19,22,25,28,31-undecaazacyclotritriacontane-2,5,8,11,14,17,20,23,26,29,32-undecone |
| (3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33-[(E,1R,2R)-1-hydroxy-2-methyl-hex-4-enyl]-6,9,18,24-tetraisobutyl-3,21-diisopropyl-1,4,7,10,12,15,19,25,28-nonamethyl-1,4,7,10,13,16,19,22,25,28,31-undecazacyclotritriacontane-2,5,8,11,14,17,20,23,26,29,32-undecone | (3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33-[(E,1R,2R)-1-hydroxy-2-methylhex-4-enyl]-1,4,7,10,12,15,19,25,28-nonamethyl-6,9,18,24-tetrakis(2-methylpropyl)-3,21-di(propan-2-yl)-1,4,7,10,13,16,19 | (3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33-[(E,1R,2R)-1-hydroxy-2-methylhex-4-enyl]-1,4,7,10,12,15,19,25,28-nonamethyl-6,9,18,24-tetrakis(2-methylpropyl)-3,21-di(propan-2-yl)-1,4,7,10,13,16,19,22,25,28,31-undecazacyclotritriacontane-2,5,8,11,14,17,20,23,26,29,32-undecone |
| (R-(R*,R*-(E)))-Cyclic(L-alanyl-D-alanyl-N-methyl-L-leucyl-N-methyl-L-leucyl-N-methyl-L-valyl-3-hydroxy-N,4-dimethyl-L-2-amino-6-octenoyl-L-alpha-aminobutyryl-N-methylglycyl-N-methyl-L-leucyl-L-valyl-N-methyl-L-leucyl) | (R-[R*,R*-(E)])-Cyclic(L-alanyl-D-alanyl-N-methyl-L-leucyl-N-methyl-L-leucyl-N-methyl-L-valyl-3-hydroxy-N,4-dimethyl-L-2-amino-6-octenoyl-L-alpha-aminobutyryl-N-methylglycyl-N-methyl-L-leucyl-L-valyl-N-methyl-L-leucyl) | 1,11-cyclo[L-alanyl-D-alanyl-N-methyl-L-leucyl-N-methyl-L-leucyl-N-methyl-L-valyl-(E)-(2S,3R,4R)-2-amino-3-hydroxy-N,4-dimethyloct-6-enoyl-L-2-aminobutanoyl-N-methylglycyl-N-methyl-L-leucyl-L-valyl-N-methyl-L-leucine] |
| 1c5f | 1cyn | 2wfj |
| 2z6w | 30-Ethyl-33-((E)-1-hydroxy-2-methyl-hex-4-enyl)-6,9,18,24-tetraisobutyl-3,21-diisopropyl-1,4,7,10,12,15,19,28-octamethyl-1,4,7,10,13,16,19,22,25,28,31undecaaza-cyclotritriacontan-2,5,8,11,14,17,20,23,26,29,32-undecaone | 30-Ethyl-33-(1-hydroxy-2-methyl-hex-4-enyl)-6,9,18,24-tetraisobutyl-3,21-diisopropyl-1,4,7,10,12,15,19,25,28-nonamethyl-1,4,7,10,13,16,19,22,25,28,31undecaaza-cyclotritriacontan-2,5,8,11,14,17,20,23,26,29,32-undecaone |
| 30-ethyl-33-[(4E)-1-hydroxy-2-methylhex-4-en-1-yl]-1,4,7,10,12,15,19,25,28-nonamethyl-6,9,18,24-tetrakis(2-methylpropyl)-3,21-bis(propan-2-yl)-1,4,7,10,13,16,19,22,25,28,31-undecaazacyclotritriacontane-2,5,8,11,14,17,20,23,26,29,32-undecone | 30-ethyl-33-[(4E)-1-hydroxy-2-methylhex-4-en-1-yl]-6,9,18,24-tetraisobutyl-3,21-diisopropyl-1,4,7,10,12,15,19,25,28-nonamethyl-1,4,7,10,13,16,19,22,25,28,31-undecaazacyclotritriacontane-2,5,8,11,14,17,20,23,26,29,32-undecone | 4jjm |
| 5946-EP0930075A1 | 5946-EP2289877A1 | 5946-EP2292601A1 |
| 5946-EP2292602A1 | 5946-EP2292603A1 | 5946-EP2292604A2 |
| 5946-EP2292618A1 | 5946-EP2293650A1 | 5946-EP2298737A1 |
| 5946-EP2298739A1 | 5946-EP2298740A1 | 5946-EP2298741A1 |
| 5946-EP2298771A2 | 5946-EP2301920A1 | 5946-EP2301940A1 |
| 5946-EP2308843A1 | 5946-EP2308861A1 | 59865-13-3 |
| 79217-60-0 | 83HN0GTJ6D | 865C133 |
| AB0090560 | AKOS015969287 | AOB2581 |
| AT-12519 | ATH-002 | Abrammune |
| Antibiotic S 7481F1 | Arpimune ME | BDBM50022815 |
| BPBio1_000496 | BRD-K03222093-001-01-8 | BRD-K13533483-001-03-0 |
| BSPBio_000450 | C042 | C62H111N11O12 |
| CAS-59865-13-3 | CB-01-09 MMX | CCG-208184 |
| CCRIS 1590 | CHEBI:4031 | CHEBI:92233 |
| CHEMBL160 | CS-2761 | CSA |
| CYA | Certified Reference Material | CicloMulsion |
| Cicloral (antibiotic) | Ciclosporin | Ciclosporin (Ciclosporin A) |
| Ciclosporin DT | Ciclosporin [INN] | Ciclosporin for system suitability, European Pharmacopoeia (EP) Reference Standard |
| Ciclosporin, European Pharmacopoeia (EP) Reference Standard | Ciclosporina | Ciclosporina Germed |
| Ciclosporina [INN-Spanish] | Ciclosporine | Ciclosporine [INN-French] |
| Ciclosporinum | Ciclosporinum [INN-Latin] | Cipol N |
| Cipol-N | Consupren | Consupren S |
| CsA & IFN.alpha. | Cyclo(((E)-(2S,3R,4R)-3-hydroxy-4-methyl-2-(methylamino)-6-octenoyl)-L-2-aminobutyryl-N-methylglycyl-N-methyl-L-leucyl-L-valyl-N-methyl-L-leucyl-L-alanyl-D-alanyl-N-methyl-L-leucyl-N-methyl-L-leucyl-N-methyl-L-valyl) | Cyclo(L-alanyl-D-alanyl-N-methyl-L-leucyl-N-methyl-L-leucyl-N-methyl-L-valyl-((3R,4R,6E)-6,7-didehydro-3-hydroxy-N,4-dimethyl-L-2-aminooctanoyl)-L-2-aminobutanoyl-N-methylglycyl-N-methyl-L-leucyl-L-valyl-N-methylleucyl) |
| Cyclo(L-alanyl-D-alanyl-N-methyl-L-leucyl-N-methyl-L-leucyl-N-methyl-L-valyl-((3R,4R,6E)-6,7-didehydro-3-hydroxy-N,4-dimethyl-L-2-aminooctanoyl-L-2-aminobutanoyl-N-methylglycyl-N-methyl-L-leucyl-L-valyl-N-methylleucyl) | Cyclo[[(E)-(2S,3R,4R)-3-hydroxy-4-methyl-2-(methylamino)-6-octenoyl]-L-2-aminobutyryl-N-methylglycyl-N-methyl-L-leucyl-L-valyl-N-methyl-L-leucyl-L-alanyl-D-alanyl-N-methyl-L-leucyl-N-methyl-L-leucyl-N-methyl-L-valyl] | Cyclokat |
| Cyclosporin | Cyclosporin A & IFN.alpha. | Cyclosporin A, >=98.5% (TLC) |
| Cyclosporin A, VETRANAL(TM), analytical standard | Cyclosporin A, from Tolypocladium inflatum, >=95% (HPLC), solid | Cyclosporin A, from Tolypocladium inflatum, BioReagent, for molecular biology, >=95% |
| Cyclosporin-A(Cyclosporine-A) | CyclosporinA | Cyclosporine A |
| Cyclosporine [USAN:USP] | Cyclosporine, Pharmaceutical Secondary Standard | Cyclosporine, United States Pharmacopeia (USP) Reference Standard |
| DB00091 | DE-076 | DRG-0275 |
| DSSTox_CID_365 | DSSTox_GSID_20365 | DSSTox_RID_75541 |
| DTXSID0020365 | Debio088 | EBD2126862 |
| Equoral | GTPL1024 | Gengraf |
| HMS1569G12 | HMS2089A09 | HMS2096G12 |
| HMS2230M14 | HMS3713G12 | HSDB 6881 |
| HY-B0579 | Ikervis (opthalmic solution) | Imusporin |
| J10181 | J90049 | KS-1257 |
| MFCD00274558 | MLS000028376 | MLS001333756 |
| MLS002153454 | MLS002207033 | Mitogard |
| Modusik-A | NCGC00016890-01 | NCGC00093704-12 |
| NCGC00164258-01 | NCGC00164258-02 | NCGC00164258-03 |
| NCGC00255232-01 | NSC 290193 | NSC290193 |
| Neoplanta | Neoral | NeuroSTAT |
| Nova-22007 | OL 27-400 | OL-27400 |
| OLO-400 | Optimmune | PMATZTZNYRCHOR-CGLBZJNRSA-N |
| Papilock | Papilock Mini | Prestwick2_000435 |
| Prestwick3_000435 | Prestwick_731 | Pulminiq |
| Q-200913 | Q367700 | Ramihyphin A |
| Restasis | S 7481F1 | S-Neoral |
| SBI-0050230.P003 | SCHEMBL3491 | SCHEMBL4442 |
| SDZ-OXL 400 | SMR000058578 | SR-01000780563 |
| SR-01000780563-3 | ST-603 | Sandimmun |
| Sandimmun Neoral | Sandimmune | Sandimmune Neoral |
| Sang 35 | Sang-2000 | Sang-35 |
| SangCyA | Seciera | Sigmasporin |
| Sigmasporin Microoral | Tox21_110667 | Tox21_110667_1 |
| Tox21_301849 | UNII-83HN0GTJ6D | Vekacia |
| Verkazia (opthalmic solution) | Zinograf ME | Zyclorin |
| cyclosporin A | cyclosporine |
| DrugBank Name | Cyclosporine |
| DrugBank | DB00091 |
| CAS Number | 104250-72-8, 55126-45-9, 59865-13-3, 79217-60-0 |
| PubChem Compound | 5284373 |
| KEGG Compound ID | C05086 |
| KEGG Drug | D00184 |
| PubChem.Substance | 46508198 |
| ChEBI | 4031 |
| PharmGKB | PA449167 |
| ChemSpider | 4447449 |
| BindingDB | 50022815.0 |
| TTD | DAP000014 |
| Wikipedia | Ciclosporin |