D0402 | Teriflunomide
L
L04AA31 Teriflunomide
[L04AA] Selective immunosuppressants
[L04A] IMMUNOSUPPRESSANTS
[L04] IMMUNOSUPPRESSANTS
[L] Antineoplastic and immunomodulating agents
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| Dihydroorotate dehydrogenase | inhibit | 295 | ||||||
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Warning |
Aggregated GHS information provided by 48 companies from 6 notifications to the ECHA C&L Inventory. Reported as not meeting GHS hazard criteria by 1 of 48 companies. For more detailed information, please visit ECHA C&L website Of the 5 notification(s) provided by 47 of 48 companies with hazard statement code(s): H302 (95.74%): Harmful if swallowed [Warning Acute toxicity, oral] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P264, P270, P301+P312, P330, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | oral | 4438mg/kg (4438mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 39, Pg. 95, 1990. | |
| rat | LD50 | intraperitoneal | 705mg/kg (705mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 39, Pg. 95, 1990. | |
| mouse | LD50 | oral | 3gm/kg (3000mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 39, Pg. 95, 1990. | |
| dog | LD50 | oral | > 5gm/kg (5000mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 39, Pg. 95, 1990. | |
| mouse | LD50 | subcutaneous | 1009mg/kg (1009mg/kg) | behavioral: muscle contraction or spasticity) | Oyo Yakuri. Pharmacometrics. Vol. 39, Pg. 95, 1990. |
| women | LDLo | oral | 108mg/kg/77W- (108mg/kg) | Australian and New Zealand Journal of Medicine. Vol. 25, Pg. 745, 1995. | |
| rat | LD50 | subcutaneous | 672mg/kg (672mg/kg) | behavioral: muscle contraction or spasticity) | Oyo Yakuri. Pharmacometrics. Vol. 39, Pg. 95, 1990. |
| mouse | LD50 | intraperitoneal | 798mg/kg (798mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 39, Pg. 95, 1990. | |
| women | TDLo | oral | 2800ug/kg/7D- (2.8mg/kg) | Medical Journal of Australia. Vol. 155, Pg. 61, 1991. | |
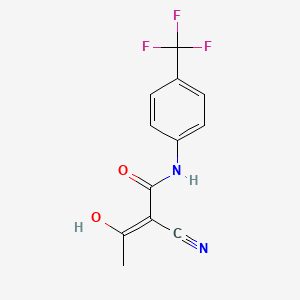
| (2Z)-2-Cyano-3-hydroxy-N-[4-(trifluoromethyl)phenyl]but-2-enamide (Teriflunomide) | (2Z)-2-[hydroxy-[4-(trifluoromethyl)anilino]methylidene]-3-oxobutanenitrile | (2Z)-3-oxidanylidene-2-[oxidanyl-[[4-(trifluoromethyl)phenyl]amino]methylidene]butanenitrile |
| (2z)-2-Cyano-3-Hydroxy-N-[4-(Trifluoromethyl)phenyl]but-2-Enamide | (Z)-2-Cyano-3-hydroxy-N-(4-(trifluoromethyl)phenyl)but-2-enamide | (Z)-2-Cyano-3-hydroxy-N-[4-(trifluoromethyl)phenyl]-2-butenamide |
| (Z)-2-Cyano-alpha'alpha'alpha-trifluoro-3-hydroxy-p-crotonotoluidide | (Z)-2-cyano-3-hydroxy-N-[4-(trifluoromethyl)phenyl]but-2-enamide | (Z)-2-cyano-alpha,alpha,alpha-trifluoro-3-hydroxy-p-crotonotoluidide |
| 108605-62-5 | 1185240-22-5 | 163451-81-8 |
| 163451-81-8 (Z Isomer) , 108605-62-5 (E/Z Mixture) | 1C058IKG3B | 2-Butenamide, 2-cyano-3-hydroxy-N-(4-(trifluoromethyl)phenyl)- |
| 2-Butenamide, 2-cyano-3-hydroxy-N-(4-(trifluoromethyl)phenyl)-, (Z)- | 2-Butenamide, 2-cyano-3-hydroxy-N-[4-(trifluoromethyl)phenyl]- | 2-Butenamide, 2-cyano-3-hydroxy-N-[4-(trifluoromethyl)phenyl]-, (2Z)- |
| 2-Cyano-3-OH-N-(4-trifluoromethylphenyl)croton amide | 2-Cyano-3-hydroxy-N-(4-(trifluoromethyl)phenyl)-2-butenamide | 2-Cyano-3-hydroxy-N-[4-(trifluoromethyl)phenyl]-2-beuteamide |
| 2-cyano-3-hydroxy-N-(4-(trifluoromethyl)phenyl)but-2-enamide | 2-cyano-3-hydroxy-n-[4-(trifluoromethyl)phenyl]-2-butenamide | 2-hydroxyethylidene-cyanoacetic acid-4-trifluoromethyl anilide |
| A 1726 | A 77-1726 | A 77-1726;A771726;HMR1726;CAS# 108605-62-5 |
| A 771726 | A-771726 | A26 |
| A77 1726 | A77-1726 | A771726 |
| A801897 | AB01565775_02 | AC-26446 |
| AKOS015994773 | API0000341 | Active metabolite of leflunomide |
| Aubagio | Aubagio (TN) | BDBM50018011 |
| C-16971 | CC-10329 | CHEBI:68540 |
| Cyano Keto leflunomide impurity | D10172 | DB08880 |
| DTXSID80893457 | EN300-189832 | Flucyamide |
| GTPL6844 | HMR 1726 | HMR-1726 |
| HMR1726 | J-010046 | LE-0275 |
| LS-46899 | Leflunomide EP Impurity B | Leflunomide Related Compound B, United States Pharmacopeia (USP) Reference Standard |
| Leflunomide USP RC B | Leflunomide-d4 Metabolite (Teriflunomide-d4) | Malononitrilamide |
| N-(4-Trifluoromethylphenyl)-2-cyano-2-hydroxycrotonamide | N-(4-Trifluoromethylphenyl)-2-cyano-3-hydroxycrotonamide | N-(4-trifluoromethylphenyl)-2-cyano-3-hydroxycrotonamide |
| N-[4-(Trifluoromethyl)phenyl]-2-cyano-3-hydroxycrotonamide | Q3077133 | RS 61980 |
| RS-61980 | RT-011141 | SB16822 |
| SC-90977 | SCHEMBL22661 | ST24036515 |
| SU 20 | SU-0020 | SW219377-1 |
| Teriflunamide | Teriflunomide | Teriflunomide |
| Teriflunomide (USAN) | Teriflunomide [INN] | Teriflunomide [USAN:INN] |
| Teriflunomide(A-771726) | Teriflunomide(Random Configuration) | Teriflunomide, A77 1726 |
| UNII-1C058IKG3B | UTNUDOFZCWSZMS-YFHOEESVSA-N | ZINC13512456 |
| s4169 | teriflunomida | teriflunomidum |
| DrugBank Name | Teriflunomide |
| DrugBank | DB08880 |
| CAS Number | 108605-62-5, 1185240-22-5, 163451-81-8, 79902-63-9 |
| PubChem Compound | 54684141 |
| KEGG Drug | D10172 |
| PubChem.Substance | 347827807 |
| ChEBI | 68540 |
| ChemSpider | 16737143 |
| BindingDB | 50018011.0 |
| Wikipedia | Teriflunomide |
| HET | A26 |
| DPD | 22169 |