Compound
D0516 | stigmatellin
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| ELECTRON TRANSPORT CHAIN | affect | 53 | ||||||
| ELECTRON TRANSPORT CHAIN | 100 mM | incubated overnight | bovine | isolated heart mitochondria | Measurements of redox potentials of the ISP | affect | 83 | |
| ELECTRON TRANSPORT CHAIN | 100 nmol/mg | bovine | mitochondria | NADH–Q | decrease | IC50 | 99 | |
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| NADH:ubiquinone reductase | 100 nmol/mg | bovine | mitochondria | NADH–Q | inhibitor | IC50 | 99 | |
| quinol | antagonist | 53 | ||||||
| Cytochrome b | 185 | |||||||
| Qo site (Qp site or ubiquinol oxidation site) | 100 mM | incubated overnight | bovine | isolated heart mitochondria | Measurements of redox potentials of the ISP | fixed conformation | 83 | |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
 |
Danger |
The GHS information provided by 1 company from 1 notification to the ECHA C&L Inventory. H300 (100%): Fatal if swallowed [Danger Acute toxicity, oral] |
P264, P270, P301+P310, P321, P330, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 30mg/kg (30mg/kg) | Journal of Antibiotics. Vol. 37, Pg. 454, 1984. | |
| mouse | LD50 | subcutaneous | 2mg/kg (2mg/kg) | Journal of Antibiotics. Vol. 37, Pg. 454, 1984. | |
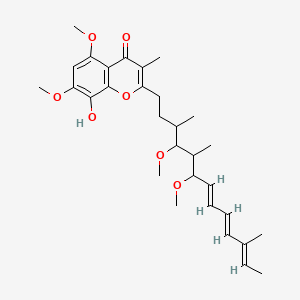
| 2-((7E,9E,11E)-4,6-dimethoxy-3,5,11-trimethyltrideca-7,9,11-trienyl)-8-hydroxy-5,7-dimethoxy-3-methyl-4H-chromen-4-one; | 91682-96-1 | SCHEMBL14066517 |
| stigmatellin |