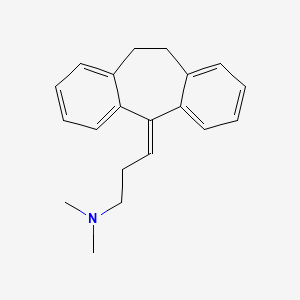
D0006 | Amitriptyline
N
N06CA01 Amitriptyline and psycholeptics
[N06CA] Antidepressants in combination with psycholeptics
[N06C] PSYCHOLEPTICS AND PSYCHOANALEPTICS IN COMBINATION
[N06] PSYCHOANALEPTICS
[N] Nervous system
N06AA09 Amitriptyline
[N06AA] Non-selective monoamine reuptake inhibitors
[N06A] ANTIDEPRESSANTS
[N06] PSYCHOANALEPTICS
[N] Nervous system
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| UNCOUPLING | rat | isolated liver mitochondria | measurements of mitochondrial respiration; RST inhibition assay, RST uncoupling assay; IC 50ratio of glucose/galactose assay | Negative | 53 | |||
| ELECTRON TRANSPORT CHAIN | pig | brain mitochondria | decrease | 60 | ||||
| ELECTRON TRANSPORT CHAIN | rat | isolated liver mitochondria | measurements of mitochondrial respiration; RST inhibition assay, RST uncoupling assay; IC 50ratio of glucose/galactose assay | Negative | 53 | |||
| GLUCOSE GALACTOSE IC50 RATIO | 70.9 ± 16.8, 72.0 ± 5.3, 1, 68.1 ± 9.1, 67.0 ± 11.6, 1 | 4hr | H9c2 cells | high-glucose–galactose cell viability assay with JC-1 mitochondrial membrane potential and ATP-depletion assays (CellTiter-Glo reagent ). | glucose/galactose IC50 ratio (JC-1 IC50 in glucose, JC-1 IC50 in galactose, JC-1 glu/gla, ATP IC50 in glucose, ATP IC50 in galactose, ATP glu/gla ) | 50 | ||
| ATP LEVEL | 197 | |||||||
| ROS PRODUCTION | 197 | |||||||
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
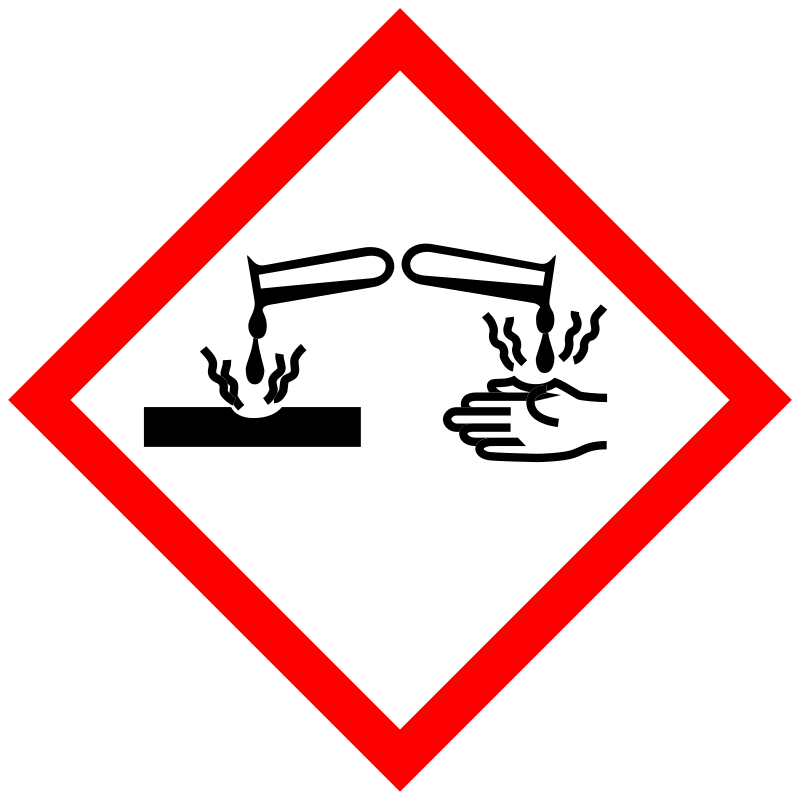     |
Danger |
Aggregated GHS information provided by 196 companies from 4 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H301+H311+H331 (80.1%): Toxic if swallowed, in contact with skin or if inhaled [Danger Acute toxicity, oral acute toxicity, dermal acute toxicity, inhalation] H302 (19.39%): Harmful if swallowed [Warning Acute toxicity, oral] H318 (80.1%): Causes serious eye damage [Danger Serious eye damage/eye irritation] H361 (80.1%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] H410 (80.1%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P261, P264, P270, P271, P273, P280, P281, P301+P310, P301+P312, P302+P352, P304+P340, P305+P351+P338, P308+P313, P310, P311, P312, P321, P322, P330, P361, P363, P391, P403+P233, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
  |
Danger |
H301: Toxic if swallowed [Danger Acute toxicity, oral] H361: Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] |
P201, P202, P264, P270, P281, P301+P310, P308+P313, P321, P330, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD50 | intramuscular | > 23mg/kg (23mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 6, Pg. 899, 1972. | |
| women | TDLo | oral | 10mg/kg (10mg/kg) | behavioral: "hallucinations, distorted perceptions" | Canadian Medical Association Journal. Vol. 129, Pg. 1203, 1983. |
| child | TDLo | oral | 58mg/kg (58mg/kg) | Southern Medical Journal. Vol. 82, Pg. 1588, 1989. | |
| monkey | LDLo | intravenous | 20300ug/kg (20.3mg/kg) | Indian Journal of Experimental Biology. Vol. 22, Pg. 539, 1984. | |
| women | TDLo | oral | 84mg/kg (84mg/kg) | cardiac: ekg changes not diagnostic of above | Japanese Journal of Toxicology. Vol. 5, Pg. 69, 1992. |
| man | TDLo | oral | 1564mg/kg/2Y- (1564mg/kg) | Italian Journal of Neurological Sciences. Vol. 9, Pg. 89, 1988. | |
| guinea pig | LDLo | intravenous | 35mg/kg (35mg/kg) | Drugs of the Future. Vol. 8, Pg. 949, 1983. | |
| man | LDLo | oral | 29mg/kg (29mg/kg) | Human & Experimental Toxicology. Vol. 9, Pg. 257, 1990. | |
| man | TDLo | oral | 714ug/kg/1D-I (0.714mg/kg) | Human Psychopharmacology. Vol. 7, Pg. 273, 1992. | |
| guinea pig | LDLo | intravenous | 52mg/kg (52mg/kg) | cardiac: other changes | Therapie. Vol. 20, Pg. 67, 1965. |
| women | TDLo | intramuscular | 240ug/kg/36H- (0.24mg/kg) | behavioral: convulsions or effect on seizure threshold | Lancet. Vol. 1, Pg. 390, 1968. |
| rat | LD50 | oral | 240mg/kg (240mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 6, Pg. 889, 1972. | |
| child | LDLo | oral | 62500ug/kg (62.5mg/kg) | New England Journal of Medicine. Vol. 267, Pg. 1031, 1962. | |
| mouse | LD50 | intravenous | 16mg/kg (16mg/kg) | behavioral: changes in motor activity (specific assay) | Journal of Medicinal Chemistry. Vol. 17, Pg. 65, 1974. |
| dog | LDLo | oral | 200mg/kg (200mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 6, Pg. 889, 1972. | |
| cat | LD50 | oral | 37mg/kg (37mg/kg) | Drug Development Research. Vol. 22, Pg. 385, 1991. | |
| women | LDLo | oral | 19mg/kg (19mg/kg) | Lancet. Vol. 2, Pg. 543, 1963. | |
| mouse | LD50 | intravenous | 21mg/kg (21mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 21, Pg. 808, 1971. | |
| rat | LD50 | intraperitoneal | 67mg/kg (67mg/kg) | Drugs in Japan Vol. -, Pg. 49, 1990. | |
| human | TDLo | oral | 2mg/kg/3D-I (2mg/kg) | British Journal of Clinical Pharmacology. Vol. 49, Pg. 118, 2000. | |
| man | TDLo | oral | 14286ug/kg (14.286mg/kg) | Lancet. Vol. 2, Pg. 543, 1963. | |
| human | TDLo | oral | 200mg/kg (200mg/kg) | JAMA, Journal of the American Medical Association. Vol. 230, Pg. 1405, 1974. | |
| man | TDLo | unreported | 45mg/kg/21D (45mg/kg) | Lancet. Vol. 2, Pg. 1202, 1972. | |
| man | TDLo | oral | 60mg/kg/3W-I (60mg/kg) | Journal of Clinical Psychiatry. Vol. 53, Pg. 160, 1992. | |
| mouse | LD50 | oral | 99mg/kg (99mg/kg) | Doklady Akademii Nauk SSSR. Proceedings of the Academy of Sciences of the USSR. For English translation, see DBIOAM and DKBSAS. Vol. 335, Pg. 388, 1994. | |
| dog | LDLo | subcutaneous | 50mg/kg (50mg/kg) | Pharmacologist. Vol. 11, Pg. 283, 1969. | |
| child | LDLo | oral | 62500ug/kg (62.5mg/kg) | American Journal of Diseases of Children. Vol. 106, Pg. 501, 1963. | |
| rat | LD50 | intravenous | 14mg/kg (14mg/kg) | "Psychotropic Drugs and Related Compounds," 2nd ed., Usdin, E., and D.H. Efron, Washington, DC, 1972Vol. -, Pg. 61, 1972. | |
| mouse | LD50 | subcutaneous | 100mg/kg (100mg/kg) | United States Patent Document. Vol. #4605673, | |
| women | TDLo | oral | 60mg/kg (60mg/kg) | Journal of Toxicology, Clinical Toxicology. Vol. 19, Pg. 67, 1982. | |
| mouse | LD50 | subcutaneous | 80mg/kg (80mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 6, Pg. 889, 1972. | |
| women | TDLo | oral | 18500ug/kg (18.5mg/kg) | Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 35, Pg. 795, 1993. | |
| rabbit | LD50 | intraperitoneal | 58700ug/kg (58.7mg/kg) | lungs, thorax, or respiration: cyanosis | Journal of Toxicology, Clinical Toxicology. Vol. 19, Pg. 321, 1982. |
| child | TDLo | oral | 4500ug/kg (4.5mg/kg) | behavioral: sleep | British Medical Journal. Vol. 3, Pg. 663, 1967. |
| mouse | LD50 | intraperitoneal | 48400ug/kg (48.4mg/kg) | Farmakologiya i Toksikologiya Vol. 53(4), Pg. 19, 1990. | |
| rabbit | LD50 | intravenous | 8600ug/kg (8.6mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 15, Pg. 863, 1965. | |
| mouse | LD50 | intraperitoneal | 65mg/kg (65mg/kg) | Journal of Drug Research. Vol. 16, Pg. 7, 1985. | |
| mouse | LD50 | oral | 140mg/kg (140mg/kg) | Wiener Medizinische Wochenschrift. Vol. 111, Pg. 256, 1961. | |
| dog | LD50 | intravenous | > 27mg/kg (27mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 6, Pg. 899, 1972. | |
| child | TDLo | oral | 4167ug/kg (4.167mg/kg) | New England Journal of Medicine. Vol. 267, Pg. 1031, 1962. | |
| infant | TDLo | oral | 50mg/kg (50mg/kg) | American Journal of Diseases of Children. Vol. 130, Pg. 507, 1976. | |
| rat | LD50 | subcutaneous | 385mg/kg (385mg/kg) | European Journal of Pharmacology. Vol. 32, Pg. 108, 1975. | |
| rabbit | LD50 | intravenous | 9900ug/kg (9.9mg/kg) | "Psychotropic Drugs and Related Compounds," 2nd ed., Usdin, E., and D.H. Efron, Washington, DC, 1972Vol. -, Pg. 61, 1972. | |
| mouse | LD50 | unreported | 80mg/kg (80mg/kg) | autonomic nervous system: "smooth muscle relaxant (mechanism undefined, spasmolytic)" | Journal of Medicinal Chemistry. Vol. 10, Pg. 418, 1967. |
| women | TDLo | oral | 16800ug/kg/2W (16.8mg/kg) | Lancet. Vol. 1, Pg. 426, 1968. | |
| rat | LD50 | oral | 320mg/kg (320mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 15, Pg. 863, 1965. | |
| human | TDLo | oral | 14mg/kg (14mg/kg) | cardiac: other changes | Proceedings of the European Society for the Study of Drug Toxicity. Vol. 6, Pg. 171, 1965. |
| rat | LD50 | intraperitoneal | 72mg/kg (72mg/kg) | Polish Journal of Pharmacology and Pharmacy. Vol. 27, Pg. 503, 1975. | |
| women | TDLo | oral | 13500ug/kg/3D (13.5mg/kg) | Lancet. Vol. 1, Pg. 390, 1968. | |
| (3-(5,6-dihydrodibenzo[b,f][7]annulen-11-ylidene)propyl)dimethylamine | 1-Propanamine, 3-(10,11-dihydro-5H-dibenzo(a,d)cyclohepten-5-ylidene)-N,N-dimethyl- | 1-Propanamine, 3-(10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-ylidene)-N,N-dimethyl- |
| 10,11-Dihydro-5-(.gamma.-dimethylaminopropylidene)-5H-dibenzo(a,d)cycloheptene | 10,11-Dihydro-5-(gamma-dimethylaminopropylidene)-5H-dibenzo(a,d)cycloheptene | 10,11-Dihydro-N,N-dimethyl-5H-dibenzo(a,d)heptalene-.DELTA.5,.gamma.-propylamine |
| 10,11-Dihydro-N,N-dimethyl-5H-dibenzo(a,d)heptalene-delta(5),gamma-propylamine | 10,11-Dihydro-N,N-dimethyl-5H-dibenzo(a,d)heptalene-delta(sup 5),gamma-propylamine | 10,11-dihydro-5-(gamma-dimethylaminopropylidene)-5H-dibenzo[a,d]cycloheptene |
| 10,11-dihydro-N,N-dimethl-5H-dibenzo[a,d]cycloheptene-(delta(5, gamma))-propylamine | 1409-EP2269989A1 | 1409-EP2275420A1 |
| 1409-EP2277861A1 | 1409-EP2298313A1 | 1409-EP2298731A1 |
| 1409-EP2298764A1 | 1409-EP2298765A1 | 1409-EP2305652A2 |
| 1409-EP2305669A1 | 1409-EP2314585A1 | 1806D8D52K |
| 3-(10,11-Dihydro-5H-dibenzo(a,d)cyclohepten-5-yliden)-N,N-dimethylpropylamin | 3-(10,11-Dihydro-5H-dibenzo(a,d)cyclohepten-5-ylidene)-N,N-dimethyl-1-propanamine | 3-(10,11-Dihydro-5H-dibenzo-[a,d]cyclohepten-5-ylidene)-N,N-dimethyl-1-propanamine, hydrochloride |
| 3-(10,11-Dihydro-5H-dibenzo[a,d][7]annulen-5-ylidene)-N,N-dimethyl-1-propanamine | 3-(10,11-Dihydro-5H-dibenzo[a,d]cyclohepten-5-ylidene)-N,N-dimethyl-1-propanamine | 3-(10,11-dihydro-5H-dibenzo[a,d][7]annulen-5-ylidene)-N,N-dimethylpropan-1-amine |
| 3-(10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-ylidene)-N,N-dimethylpropan-1-amine | 3-(5,6-dihydrodibenzo[[?],[?]][7]annulen-11-ylidene)-N,N-dimethyl-propan-1-amine | 412A072 |
| 5-(.gamma.-Dimethylaminopropylidene)-10,11-dihydro-5H-dibenzo(a,d)cycloheptene | 5-(.gamma.-Dimethylaminopropylidene)-5H-Dibenzo[a,d][1,4]cycloheptadiene | 5-(.gamma.-Dimethylaminopropylidene)-5H-dibenzo(a,d)-10,11-dihydrocycloheptene |
| 5-(.gamma.-Dimethylaminopropylidine)-5H-dibenzo(a,d)(1,4)cycloheptadiene | 5-(3'-Dimethylaminopropylidene)-dibenzo-(a,d)(1,4)-cycloheptadiene | 5-(3'-Dimethylaminopropylidene)-dibenzo-[a,d][1,4]-cycloheptadiene |
| 5-(3-Dimethylaminopropylidene)-10,11-dihydro-5H-dibenzo(a,d)cycloheptatriene | 5-(3-Dimethylaminopropylidene)-10,11-dihydro-5H-dibenzo(a,d)cycloheptene | 5-(3-Dimethylaminopropylidene)-5H-dibenzo[a,d]-10,11-dihydrocycloheptene |
| 5-(3-dimethylaminopropylidene)dibenzo[a,d][1,4]-cycloheptadiene | 5-(gamma-Dimethylaminopropylidene)-10,11-dihydro-5H-dibenzo(a,d)cycloheptene | 5-(gamma-Dimethylaminopropylidene)-5H-Dibenzo[a,d][1,4]cycloheptadiene |
| 5-(gamma-Dimethylaminopropylidene)-5H-dibenzo(a,d)(1,4)cycloheptadiene | 5-(gamma-Dimethylaminopropylidene)-5H-dibenzo[a,d]10,11-dihydrocycloheptene | 5-(gamma-Dimethylaminopropylidine)-5H-dibenzo(a,d)(1,4)cycloheptadiene |
| 50-48-6 | 5H-Dibenzo(a,d)cycloheptene-.delta.(sup 5),.gamma.-propylamine, 10,11-dihydro-N,N-dimethyl- | 5H-Dibenzo(a,d)cycloheptene-delta(sup 5),gamma-propylamine, 10,11-dihydro-N,N-dimethyl- |
| 5H-Dibenzo(a,d)cycloheptene-delta5,gamma-propylamine, 10,11-dihydro-N,N-dimethyl- | 5H-Dibenzo[a,d]cycloheptene-.delta.5,.gamma.-propylamine, 10,11-dihydro-N,N-dimethyl- | 5H-Dibenzo[a,d]cycloheptene-Delta5,gamma-propylamine, 10,11-dihydro-N,N-dimethyl- (6CI,8CI) 5-(gamma-Dimethylaminopropylidene)-10,11-dihydro-5H-dibenzo[a,d]cycloheptene |
| AB00053417-12 | AB00053417_13 | AB00053417_14 |
| AB00514631 | AKOS000512694 | API0001457 |
| ARONIS24381 | Adepress | Adepril |
| Amitril | Amitriprolidine | Amitriptilina |
| Amitriptilina [INN-Spanish] | Amitriptilina [Italian] | Amitriptylin |
| Amitriptylin [German] | Amitriptyline (INN) | Amitriptyline [INN:BAN] |
| Amitriptylinum | Amitriptylinum [INN-Latin] | Amitryptiline |
| Amitryptyline | Amytriptiline | Amytriptylin |
| BBC/541 | BBL028529 | BCP09083 |
| BDBM50020712 | BIDD:GT0115 | BPBio1_000317 |
| BRD-K53737926-003-05-4 | BRD-K53737926-003-14-6 | BRN 2217885 |
| BSPBio_000287 | BSPBio_001836 | C06824 |
| CAS-549-18-8 | CCG-204207 | CCRIS 9174 |
| CHEBI:2666 | CHEMBL629 | D07448 |
| DB00321 | DTXSID7022594 | Damilan |
| Damilen | Damitriptyline | DivK1c_000766 |
| Domical (Salt/Mix) | EC 200-041-6 | EINECS 200-041-6 |
| Elanil | Elavil | FT-0653242 |
| Flavyl | GTPL200 | HSDB 3007 |
| IDI1_000766 | KBio1_000766 | KBio2_000424 |
| KBio2_002261 | KBio2_002992 | KBio2_004829 |
| KBio2_005560 | KBio2_007397 | KBio3_001336 |
| KBio3_002741 | KBioGR_000592 | KBioGR_002261 |
| KBioSS_000424 | KBioSS_002262 | KRMDCWKBEZIMAB-UHFFFAOYSA-N |
| L001041 | LS-60747 | Lantron |
| Laroxil | Laroxyl (Salt/Mix) | Laroxyl (TN) |
| Lentizol (Salt/Mix) | Limbitrol | Lopac-A-8404 |
| Lopac0_000112 | MCULE-3537115467 | MK 230 |
| MRF-0000533 | N 750 | NCGC00015095-01 |
| NCGC00015095-02 | NCGC00015095-03 | NCGC00015095-04 |
| NCGC00015095-05 | NCGC00015095-06 | NCGC00015095-07 |
| NCGC00015095-08 | NCGC00015095-09 | NCGC00015095-10 |
| NCGC00015095-11 | NCGC00015095-12 | NCGC00024433-04 |
| NCGC00183047-01 | NINDS_000766 | NSC169910 |
| Oprea1_479304 | PDSP1_001564 | PDSP2_001548 |
| Prestwick0_000074 | Prestwick1_000074 | Prestwick2_000074 |
| Prestwick3_000074 | Proheptadiene | Q58397 |
| Redomex | Ro 4-1575 | SBB080793 |
| SBI-0050100.P004 | SCHEMBL7824 | SPBio_000082 |
| SPBio_002208 | STK525215 | Sarotex |
| Seroten | Spectrum2_000101 | Spectrum3_000298 |
| Spectrum4_000146 | Spectrum5_000806 | Spectrum_000044 |
| Triptanol | Triptilin | Triptisol |
| Triptizol (Salt/Mix) | Tryptanol | UNII-1806D8D52K |
| Vanatrip (Salt/Mix) | W-109252 | ZINC968257 |
| ZX-AS004743 | amitriptyline | cMAP_000001 |
| dimethyl({3-[(2E)-tricyclo[9.4.0.0^{3,8}]pentadeca-1(15),3,5,7,11,13-hexaen-2-ylidene]propyl})amine | dimethyl({3-[(2Z)-tricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8),4,6,12,14-hexaen-2-ylidene]propyl})amine |
| DrugBank Name | Amitriptyline |
| DrugBank | DB00321 |
| CAS Number | 30227-34-0, 50-48-6, 549-18-8 |
| PubChem Compound | 2160 |
| KEGG Compound ID | C06824 |
| KEGG Drug | D07448 |
| PubChem.Substance | 46508798 |
| ChEBI | 2666 |
| PharmGKB | PA448385 |
| ChemSpider | 2075 |
| BindingDB | 50020712.0 |
| TTD | DNC001466 |
| Wikipedia | Amitriptyline |
| HET | TP0 |
| DPD | 10187|9881|5979 |