D0009 | Antimycin A
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| OXYGEN CONSUMPTION RATE (OCR) | 4±0.2 nM | 2 minutes | human | HepG2 | Measurement of OCR | decrease | EC50 | 7 |
| ELECTRON TRANSPORT CHAIN | decrease | 26 | ||||||
| ELECTRON TRANSPORT CHAIN | decrease | 35 | ||||||
| ELECTRON TRANSPORT CHAIN | bovine | isolated heart mitochondria cytochrome bc1 complex | affect | 166 | ||||
| ECAR | 100±20 nM | 2 minutes | human | HepG2 | Measurement of ECAR | increase | EC50 | 7 |
| GLUCOSE GALACTOSE IC50 RATIO | 5.9 (42.1nM/7.1nM) | 24hr | L6 | ATP levels in high-glucose (25 mM) or galactose (10 mM) medium | glucose/galactose IC50 ratio | 325 | ||
| GLUCOSE GALACTOSE IC50 RATIO | 10.7 (176.5nM/16.5nM) | 24hr | H9c2 | ATP levels in high-glucose (25 mM) or galactose (10 mM) medium | glucose/galactose IC50 ratio | 325 | ||
| GLUCOSE GALACTOSE IC50 RATIO | 17.7(58.1nM/3.3nM) | 24hr | HepG2 | ATP levels in high-glucose (25 mM) or galactose (10 mM) medium | glucose/galactose IC50 ratio | 325 | ||
| GLUCOSE GALACTOSE IC50 RATIO | 12000 | LUHMES (Lund human mesencephalic) cells | Glc–Gal–NeuriTox assay | EC25(NA) [Glc/Gal] | 326 | |||
| MITOCHONDRIAL MOTILITY | 100 μM | decrease | 217 | |||||
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| Quinol--cytochrome-c reductase | inhibitor | 26 | ||||||
| Quinol--cytochrome-c reductase | inhibitor | 35 | ||||||
| Quinol--cytochrome-c reductase | 12000 | LUHMES (Lund human mesencephalic) cells | Glc–Gal–NeuriTox assay | EC25(NA) [Glc/Gal] | 326 | |||
| Cytochrome b | 185 | |||||||
| Qi site (Qn site or quinone reduction site) | bovine | isolated heart mitochondria cytochrome bc1 complex | inhibitor | 166 | ||||
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Danger |
Aggregated GHS information provided by 192 companies from 2 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H300 (100%): Fatal if swallowed [Danger Acute toxicity, oral] H400 (100%): Very toxic to aquatic life [Warning Hazardous to the aquatic environment, acute hazard] H410 (80.21%): Very toxic to aquatic life with long lasting effects [Warning Hazardous to the aquatic environment, long-term hazard] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P264, P270, P273, P301+P310, P321, P330, P391, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 7600ug/kg (7.6mg/kg) | "Index of Antibiotics from Actinomycetes," Umezawa, H. et al., eds., Tokyo, Univ. of Tokyo Press, 1967Vol. -, Pg. 144, 1967. | |
| bird - wild | LD50 | oral | 5mg/kg (5mg/kg) | Antimicrobial Agents and Chemotherapy Vol. -, Pg. 757, 1966. | |
| rat | LD50 | oral | 1469mg/kg (1469mg/kg) | Journal of Drug Research. Vol. 7(2), Pg. 1, 1975. | |
| duck | LD50 | oral | 2900ug/kg (2.9mg/kg) | Antimicrobial Agents and Chemotherapy Vol. -, Pg. 757, 1966. | |
| rat | LD50 | intraperitoneal | 810ug/kg (0.81mg/kg) | lungs, thorax, or respiration: other changes | Cancer Research. Vol. 13, Pg. 49, 1953. |
| mouse | LD50 | intramuscular | 1gm/kg (1000mg/kg) | Journal of Drug Research. Vol. 7(2), Pg. 1, 1975. | |
| quail | LD50 | oral | 39mg/kg (39mg/kg) | Antimicrobial Agents and Chemotherapy Vol. -, Pg. 757, 1966. | |
| domestic animals - goat/sheep | LD50 | oral | > 1mg/kg (1mg/kg) | Antimicrobial Agents and Chemotherapy Vol. -, Pg. 757, 1966. | |
| mouse | LD50 | intravenous | 900ug/kg (0.9mg/kg) | "Index of Antibiotics from Actinomycetes," Umezawa, H. et al., eds., Tokyo, Univ. of Tokyo Press, 1967Vol. -, Pg. 144, 1967. | |
| mouse | LD50 | intraperitoneal | 820ug/kg (0.82mg/kg) | Journal of Antibiotics, Series A. Vol. 11, Pg. A26, 1958. | |
| mouse | LD50 | subcutaneous | 1600ug/kg (1.6mg/kg) | "Antibiotics: Origin, Nature, and Properties," Korzyoski, T., et al., eds., Washington, DC, American Soc. for Microbiology, 1978Vol. 2, Pg. 1078, 1978. | |
| rat | LD50 | oral | 28mg/kg (28mg/kg) | Antimicrobial Agents and Chemotherapy Vol. -, Pg. 757, 1966. | |
| mouse | LD50 | intravenous | 893ug/kg (0.893mg/kg) | Journal of Antibiotics, Series A. Vol. 9, Pg. 63, 1956. | |
| pigeon | LD50 | oral | 2mg/kg (2mg/kg) | Antimicrobial Agents and Chemotherapy Vol. -, Pg. 757, 1966. | |
| rabbit | LD50 | oral | 10mg/kg (10mg/kg) | Antimicrobial Agents and Chemotherapy Vol. -, Pg. 757, 1966. | |
| chicken | LD50 | oral | > 160mg/kg (160mg/kg) | Antimicrobial Agents and Chemotherapy Vol. -, Pg. 757, 1966. | |
| dog | LD50 | oral | > 5mg/kg (5mg/kg) | Antimicrobial Agents and Chemotherapy Vol. -, Pg. 757, 1966. | |
| guinea pig | LD50 | oral | 1800ug/kg (1.8mg/kg) | Antimicrobial Agents and Chemotherapy Vol. -, Pg. 757, 1966. | |
| rat | LD50 | subcutaneous | 25mg/kg (25mg/kg) | "Antibiotics: Origin, Nature, and Properties," Korzyoski, T., et al., eds., Washington, DC, American Soc. for Microbiology, 1978Vol. 2, Pg. 1078, 1978. | |
| mouse | LD50 | oral | 55mg/kg (55mg/kg) | Antimicrobial Agents and Chemotherapy Vol. -, Pg. 757, 1966. | |
| mouse | LD50 | subcutaneous | 25mg/kg (25mg/kg) | "Index of Antibiotics from Actinomycetes," Umezawa, H. et al., eds., Tokyo, Univ. of Tokyo Press, 1967Vol. -, Pg. 144, 1967. | |
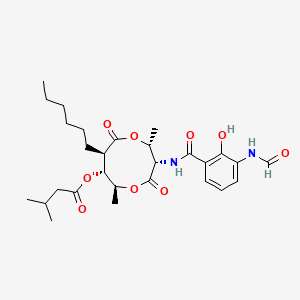
| (2R,3S,6S,7R,8R)-3-[(3-formamido-2-hydroxybenzoyl)amino]-8-hexyl-2,6-dimethyl-4,9-dioxo-1,5-dioxonan-7-yl 3-methylbutanoate | (3S,6S,2R,7R,8R)-3-[(3-carbonylamino-2-hydroxyphenyl)carbonylamino]-8-hexyl-2, 6-dimethyl-4,9-dioxo-1,5-dioxonan-7-yl 3-methylbutanoate | 116095-18-2 |
| 1397-94-0 | 3-Methylbutanoic acid 3-[[3-(formylamino)-2-hydroxybenzoyl]amino]-8-hexyl-2,6-dimethyl-4,9-dioxo-1,5-dioxonan-7-yl ester | 642-15-9 |
| 75G3NMU1TS | 8S75R39Y6J | AIDS032154 |
| AKOS015889205 | AWB | Antimycin A |
| Antimycin A1b | Antimycin-A | Antipiricullin |
| BCP9000305 | BDBM50191588 | Butanoic acid, 2(or3)-methyl-,(2R,3S,6S,7R,8R)-3-[[3-(formylamino)-2-hydroxybenzoyl]amino]-8-hexyl-2,6-dimethyl-4,9-dioxo-1,5-dioxonan-7-ylester (9CI) |
| Butanoic acid, 3-methyl-, 3-((3-(formylamino)-2-hydroxybenzoyl)amino)-8-hexyl-2,6-dimethyl-4,9-dioxo-1,5-dioxonan-7-yl ester, (2R-(2R*,3S*,6S*,7R*,8R*))- | CCG-208457 | CCRIS 924 |
| CHEBI:22584 | CHEBI:2762 | CHEMBL211501 |
| Caswell No. 052B | DTXSID3058668 | EPA Pesticide Chemical Code 006314 |
| Fintrol | HSDB 6417 | LS-21350 |
| NCGC00017338-02 | NCGC00017338-03 | NCGC00017338-04 |
| NCGC00017338-05 | NCGC00142516-01 | NCGC00142516-02 |
| NCGC00142516-03 | SCHEMBL218354 | SR-05000002233 |
| SR-05000002233-2 | SR-05000002233-3 | ST057184 |
| UNII-75G3NMU1TS | UNII-8771CCP8LT component UIFFUZWRFRDZJC-SBOOETFBSA-N | UNII-8S75R39Y6J |
| UNII-8S75R39Y6J component UIFFUZWRFRDZJC-SBOOETFBSA-N | VA10306 | Virosin |
| ZINC5224254 | [(2R,3S,6S,7R,8R)-3-[(3-formamido-2-hydroxy-benzoyl)amino]-8-hexyl-2,6-dimethyl-4,9-dioxo-1,5-dioxonan-7-yl] 3-methylbutanoate | [(2r,3s,6s,7r,8r)-3-[(3-Formamido-2-Oxidanyl-Phenyl)carbonylamino]-8-Hexyl-2,6-Dimethyl-4,9-Bis(Oxidanylidene)-1,5-Dioxonan-7-Yl] 3-Methylbutanoate |
| antimycin A1 | isovaleric acid 8-ester with 3-formamido-N-(7-hexyl-8-hydroxy-4,9-dimethyl-2,6-dioxo-1,5-dioxonan-3-yl)salicylamide |
| CAS Number | 116095-18-2, 1397-94-0, 27220-56-0, 642-15-9 |
| PubChem Compound | 14957 |
| KEGG Compound ID | C11339 |
| ChEBI | 22584 |
| ChemSpider | 14246 |
| Wikipedia | Antimycin A |