D0023 | Chloramphenicol
D
G
J
S
S03AA08 Chloramphenicol
[S03AA] Antiinfectives
[S03A] ANTIINFECTIVES
[S03] OPHTHALMOLOGICAL AND OTOLOGICAL PREPARATIONS
[S] Sensory organs
S02AA01 Chloramphenicol
[S02AA] Antiinfectives
[S02A] ANTIINFECTIVES
[S02] OTOLOGICALS
[S] Sensory organs
S01AA01 Chloramphenicol
[S01AA] Antibiotics
[S01A] ANTIINFECTIVES
[S01] OPHTHALMOLOGICALS
[S] Sensory organs
J01BA01 Chloramphenicol
[J01BA] Amphenicols
[J01B] AMPHENICOLS
[J01] ANTIBACTERIALS FOR SYSTEMIC USE
[J] Antiinfectives for systemic use
G01AA05 Chloramphenicol
[G01AA] Antibiotics
[G01A] ANTIINFECTIVES AND ANTISEPTICS, EXCL. COMBINATIONS WITH CORTICOSTEROIDS
[G01] GYNECOLOGICAL ANTIINFECTIVES AND ANTISEPTICS
[G] Genitourinary system and reproductive hormones
D10AF03 Chloramphenicol
[D10AF] Antiinfectives for treatment of acne
[D10A] ANTI-ACNE PREPARATIONS FOR TOPICAL USE
[D10] ANTI-ACNE PREPARATIONS
[D] Dermatological drugs
D06AX02 Chloramphenicol
[D06AX] Other antibiotics for topical use
[D06A] ANTIBIOTICS FOR TOPICAL USE
[D06] ANTIBIOTICS AND CHEMOTHERAPEUTICS FOR DERMATOLOGICAL USE
[D] Dermatological drugs
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| FERRITIN DEPLETION | 197 | |||||||
| MEGAMITOCHONDRIA | 200-300µg/ml | 22hr | primary culture rat hepatocytes, RL-34,COS-1, IAR-20 | induce | 292 | |||
| MITOCHONDRIAL RESPIRATORY CHAIN COMPLEX ASSEMBLY | 50 μg/ml | 5 days | 143B osteosarcoma cells | Blue Native Gel | inhibitor | 286 | ||
| MITOCHONDRIAL RESPIRATORY CHAIN COMPLEX ASSEMBLY | 50µg/mL | 4 days | complex I de novo assembly analysed by BN-PAGE | inhibitor | 287 | |||
| MITOCHONDRIAL PROTEIN TRANSLATION | 9.8 ± 0.5 μM | rat | liver mitochondria | Mitochondrial protein synthesis assay (The incorporation of [35S]methionine into mitochondrial protein was determined by a filter paper disk assay ) | IC50 | 281 | ||
| MITOCHONDRIAL PROTEIN TRANSLATION | haplo-insufficiency profiling (HIP), a well-validated chemical genomics platform developed in the yeast S. Cerevisiae. | inhibitor | 288 | |||||
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
   |
Danger |
Aggregated GHS information provided by 394 companies from 26 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. Reported as not meeting GHS hazard criteria by 6 of 394 companies. For more detailed information, please visit ECHA C&L website Of the 25 notification(s) provided by 388 of 394 companies with hazard statement code(s): H317 (11.34%): May cause an allergic skin reaction [Warning Sensitization, Skin] H318 (40.72%): Causes serious eye damage [Danger Serious eye damage/eye irritation] H350 (48.97%): May cause cancer [Danger Carcinogenicity] H351 (50.77%): Suspected of causing cancer [Warning Carcinogenicity] H361 (61.86%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P261, P272, P280, P281, P302+P352, P305+P351+P338, P308+P313, P310, P321, P333+P313, P363, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
 |
Danger |
H350: May cause cancer [Danger Carcinogenicity] |
P201, P202, P281, P308+P313, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
 |
Danger |
H303: May be harmful if swallowed [Warning Acute toxicity, oral] H340: May cause genetic defects [Danger Germ cell mutagenicity] H350: May cause cancer [Danger Carcinogenicity] H361: Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] H372: Causes damage to organs through prolonged or repeated exposure [Danger Specific target organ toxicity, repeated exposure] H373: Causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure] |
P201, P202, P260, P264, P270, P281, P308+P313, P312, P314, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | intraperitoneal | > 5gm/kg (5000mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 3, Pg. 390, 1969. | |
| rat | LD50 | intravenous | 171mg/kg (171mg/kg) | Journal of Clinical Investigation. Vol. 28, Pg. 943, 1949. | |
| mouse | LD50 | intravenous | 368mg/kg (368mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 3, Pg. 390, 1969. | |
| human | TDLo | unreported | 214mg/kg/10D (214mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 24, Pg. 944, 1974. | |
| infant | TDLo | intramuscular | 250mg/kg/2D (250mg/kg) | vascular: change in plasma or blood valume | New England Journal of Medicine. Vol. 262, Pg. 787, 1960. |
| mouse | LD50 | intraperitoneal | 1100mg/kg (1100mg/kg) | Dissertationes Pharmaceuticae. Vol. 14, Pg. 21, 1962. | |
| mouse | LD50 | intraperitoneal | > 5gm/kg (5000mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 3, Pg. 390, 1969. | |
| guinea pig | LD50 | oral | 500mg/kg (500mg/kg) | Farmaco, Edizione Scientifica. Vol. 10, Pg. 3, 1955. | |
| guinea pig | LD50 | intravenous | 560mg/kg (560mg/kg) | Farmaco, Edizione Scientifica. Vol. 9, Pg. 21, 1954. | |
| rat | LD50 | intraperitoneal | 1811mg/kg (1811mg/kg) | Toxicology and Applied Pharmacology. Vol. 18, Pg. 185, 1971. | |
| rat | LD50 | intravenous | 339mg/kg (339mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 3, Pg. 390, 1969. | |
| dog | LD | intramuscular | > 101mg/kg (101mg/kg) | Journal of Clinical Investigation. Vol. 28, Pg. 943, 1949. | |
| rat | LD50 | oral | > 5gm/kg (5000mg/kg) | Drugs in Japan Vol. -, Pg. 740, 1995. | |
| mouse | LD50 | oral | > 7gm/kg (7000mg/kg) | Oyo Yakuri. Pharmacometrics. Vol. 3, Pg. 390, 1969. | |
| mouse | LD50 | oral | 1500mg/kg (1500mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 5, Pg. 1, 1955. | |
| mouse | LD50 | intravenous | 110mg/kg (110mg/kg) | Journal of Clinical Investigation. Vol. 28, Pg. 943, 1949. | |
| rat | LD50 | subcutaneous | 5gm/kg (5000mg/kg) | gastrointestinal: "hypermotility, diarrhea" | Toxicology and Applied Pharmacology. Vol. 9, Pg. 445, 1966. |
| women | LDLo | oral | 400mg/kg (400mg/kg) | JAMA, Journal of the American Medical Association. Vol. 234, Pg. 149, 1975. | |
| infant | LDLo | unreported | 200mg/kg/4D-I (200mg/kg) | Lancet. Vol. 1, Pg. 555, 1986. | |
| rabbit | LD50 | intravenous | 117mg/kg (117mg/kg) | Journal of Clinical Investigation. Vol. 28, Pg. 943, 1949. | |
| rat | LD50 | oral | 2500mg/kg (2500mg/kg) | Farmaco, Edizione Scientifica. Vol. 10, Pg. 3, 1955. | |
| infant | TDLo | oral | 440mg/kg (440mg/kg) | JAMA, Journal of the American Medical Association. Vol. 234, Pg. 149, 1975. | |
| child | TDLo | unreported | 250mg/kg/10D (250mg/kg) | Clinical Pediatrics Vol. 14, Pg. 499, 1975. | |
| dog | LD | oral | > 300mg/kg (300mg/kg) | Journal of Clinical Investigation. Vol. 28, Pg. 943, 1949. | |
| infant | LDLo | intravenous | 30mg/kg/3D-I (30mg/kg) | Journal of Pediatrics. Vol. 103, Pg. 487, 1983. | |
| dog | LDLo | intravenous | 150mg/kg (150mg/kg) | Journal of Bacteriology. Vol. 55, Pg. 425, 1948. | |
| mouse | LD50 | subcutaneous | 400mg/kg (400mg/kg) | "Antibiotics: Origin, Nature, and Properties," Korzyoski, T., et al., eds., Washington, DC, American Soc. for Microbiology, 1978Vol. 1, Pg. 493, 1978. | |
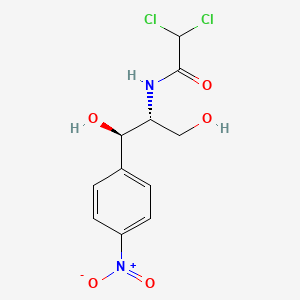
| (-)-chloramphenicol | 2,2-Dichloro-N-((1R,2R)-1,3-dihydroxy-1-(4-nitrophenyl)propan-2-yl)acetamide | 2,2-Dichloro-N-(2-hydroxy-1-(hydroxymethyl)-2-(4-(hydroxy(oxido)amino)phenyl)ethyl)acetamide, (1R, 2R)- |
| 2,2-Dichloro-N-[(1R,2R)-1,3-dihydroxy-1-(4-nitrophenyl)-2-propyl]acetamide | 2,2-Dichloro-N-[2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl]acetamide | 2,2-dichloro-N-[(1R,2R)-1,3-dihydroxy-1-(4-nitrophenyl)propan-2-yl]acetamide |
| 2,2-dichloro-N-[(1R,2R)-2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl]acetamide | 2787-09-9 | 4-13-00-02742 (Beilstein Handbook Reference) |
| 56-75-7 | 66974FR9Q1 | AB0015087 |
| AB00374860 | AB00374860-13 | AB00374860-14 |
| AB00374860_15 | AB02994 | AB1009524 |
| ACETAMIDE, 2,2-DICHLORO-N-[(1R,2R)-2-HYDROXY-1-(HYDROXYMETHYL)-2-(4-NITROPHENYL)ETHYL]- | AI3-25003 | AK174225 |
| AKOS005111001 | ARONIS23913 | AS-14683 |
| Acetamide, 2,2-dichloro-N-((1R,2R)-2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl)- | Acetamide, 2,2-dichloro-N-(.beta.-hydroxy-.alpha.-(hydroxymethyl)-p-nitrophenethyl)-, D-(-)-threo- | Acetamide, 2,2-dichloro-N-(2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl)-, (R*,R*)-(+-)- |
| Acetamide, 2,2-dichloro-N-(2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl)-, (R-(R*,R*))- | Acetamide, 2,2-dichloro-N-(2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl)-, (theta-(theta,theta))- | Acetamide, 2,2-dichloro-N-(beta-hydroxy-alpha-(hydroxymethyl)-p-nitrophenethyl)-, D-(-)-threo- |
| Acetamide, 2,2-dichloro-N-(beta-hydroxy-alpha-(hydroxymethyl)-p-nitrophenethyl)-,D-(-)-threo- | Acetamide, 2,2-dichloro-N-[.beta.-hydroxy-.alpha.-(hydroxymethyl)-p-nitrophenethyl]-, D-threo-(-)- | Acetamide, 2,2-dichloro-N-[2-hydroxy-1-(hydroxymethyl)-2-(4- nitrophenyl)ethyl]-, [R-(R*,R*)]- |
| Acetamide, 2,2-dichloro-N-[2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl]-, [R-(R*,R*)]- | Acetamide,2-dichloro-N-[.beta.-hydroxy-.alpha.-(hydroxymethyl)-p-nitrophenethyl] | Acetamide,2-dichloro-N-[.beta.-hydroxy-.alpha.-(hydroxymethyl)-p-nitrophenethyl]-, D-threo-(-)- |
| Acetamide,2-dichloro-N-[.beta.-hydroxy-.alpha.-(hydroxymethyl)-p-nitrophenethyl]-,D-(-)-threo- | Acetamide,2-dichloro-N-[2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl]-, [R-(R*,R*)]- | Ak-chlor |
| Alficetyn | Ambofen | Amphenicol |
| Amphicol | Amphicol (TN) | Amseclor |
| Anacetin | Aquamycetin | Austracil |
| Austracol | BBC/192 | BCP12150 |
| BDBM23447 | BIC0113 | BIDD:GT0145 |
| BPBio1_000135 | BRD-K08111712-001-02-7 | BRD-K08111712-001-16-7 |
| BRN 2225532 | BSPBio_000121 | Biocetin |
| Biophenicol | C-19742 | C-3307 |
| C.A.F | C00918 | C11H12Cl2N2O5 |
| CAF | CAF (pharmaceutical) | CAM |
| CAS-56-75-7 | CC-25647 | CCG-220031 |
| CCRIS 3922 | CHEBI:17698 | CHEMBL130 |
| CHLORAMPHENICOL (SEE ALSO CHLORAMPHENICOL NA SUCCINATE 982-57-0) | CHLOROPTIC S.O.P | CPD000471851 |
| CS-2207 | CTK4B4374 | Catilan |
| Ch loramex | Chemiceticol | Chemicetin |
| Chemicetina | Chlomin | Chlomycol |
| Chlora-tabs | Chloramex | Chloramfenikol |
| Chloramfenikol [Czech] | Chloramficin | Chloramfilin |
| Chloramphenicol (Chloromycetin) | Chloramphenicol (JP17/USP/INN) | Chloramphenicol 10 microg/mL in Acetonitrile. Short expiry date due to chemical nature of component(s) |
| Chloramphenicol 100 microg/mL in Ethyl acetate | Chloramphenicol [INN:BAN:JAN] | Chloramphenicol [USP:INN:BAN:JAN] |
| Chloramphenicol crystalline | Chloramphenicol(Chloromycetin) | Chloramphenicol, 98% |
| Chloramphenicol, >=98% (HPLC) | Chloramphenicol, Antibiotic for Culture Media Use Only | Chloramphenicol, BioReagent, suitable for plant cell culture |
| Chloramphenicol, Biotechnology Performance Certified, suitable for plant cell culture | Chloramphenicol, British Pharmacopoeia (BP) Reference Standard | Chloramphenicol, European Pharmacopoeia (EP) Reference Standard |
| Chloramphenicol, United States Pharmacopeia (USP) Reference Standard | Chloramphenicol, VETRANAL(TM), analytical standard | Chloramphenicol, certified reference material, TraceCERT(R) |
| Chloramphenicol, d- | Chloramphenicol, gamma-irradiated | Chloramphenicol, meets USP testing specifications |
| Chloramphenicol, puriss., 98.0-102.0% | Chloramphenicol, tested according to Ph.Eur. | Chloramphenicol,(S) |
| Chloramphenicole | Chloramphenicolum | Chloramphenicolum [INN-Latin] |
| Chloramsaar | Chlorasol | Chlorbiotic (Veterinary) |
| Chloricol | Chlormycetin R | Chlornitromycin |
| Chloro-25 vetag | Chloroamphenicol | Chlorocaps |
| Chlorocid | Chlorocid S | Chlorocide |
| Chlorocidin C | Chlorocidin C tetran | Chlorocin |
| Chlorocol | Chlorofair | Chloroject L |
| Chloromax | Chloromycetin | Chloromycetin (TN) |
| Chloromycetny | Chloromycetny [Polish] | Chloromyxin (Salt/Mix) |
| Chloronitrin | Chloroptic | Chloroptic S.O.P. |
| Chlorovules | Chlorsig | Cidocetine |
| Ciplamycetin | Cloramfen | Cloramfenicol |
| Cloramfenicol [INN-Spanish] | Cloramfenicolo | Cloramfenicolo [DCIT] |
| Cloramficin | Cloramical | Cloramicol |
| Cloramidina | Cloranfenicol | Cloroamfenicolo |
| Cloroamfenicolo [Italian] | Clorocyn | Cloromisan |
| Cloromissan | Clorosintex | Comycetin |
| Cylphenicol | D(-)-threo-2-Dichloroacetamido-1-p-nitrophenyl-1,3-propanediol | D(-)-threo-2-dichloroacetamido-1-p-nitrophen yl-propanediol |
| D(-)-threo-2-dichloroacetamido-1-p-nitrophenyl-propanediol | D(-)-threo-Chloramphenicol | D-( -)-threo-1-(p-nitrophenyl)-2-dichloroacetamido-1,3-propanediol |
| D-(-)-2,2-Dichloro-N-(.beta.-hydroxy-.alpha.-(hydroxymethyl)-p-nitrophenyl-ethyl)acetamide | D-(-)-2,2-Dichloro-N-(beta-hydroxy-alpha-(hydroxymethyl)-p-nitrophenylethyl)acetamide | D-(-)-Chloramphenicol |
| D-(-)-threo-1-(4-Nitrophenyl)-2-dichloroacetamido-1,3-propanediol | D-(-)-threo-1-(p-Nitrophenyl)-2-(dichloroacetylamino)-1,3-propanediol | D-(-)-threo-1-(p-nitrophenyl)-2-dichloroacetamido-1,3-propanediol |
| D-(-)-threo-1-p-Nitrophenyl-2-dichloracetamido-1,3-propanediol | D-(-)-threo-1-p-Nitrophenyl-2-dichloroacetylamino-1,3-propanediol | D-(-)-threo-2,2-Dichloro-N-(.beta.-hydroxy-.alpha.-(hydroxymethyl))-p-nitrophenethylacetamide |
| D-(-)-threo-2,2-Dichloro-N-(.beta.-hydroxy-.alpha.-(hydroxymethyl)-p-nitrophenyl-ethyl)acetamide | D-(-)-threo-2,2-Dichloro-N-[.beta.-hydroxy-.alpha.-(hydroxymethyl)]-p-nitrophenethylacetamide | D-(-)-threo-2,2-Dichloro-N-[beta-hydroxy-alpha-(hydroxy-methyl)-p-nitrophenethyl]acetamide |
| D-(-)-threo-2,2-Dichloro-N-[beta-hydroxy-alpha-(hydroxymethyl)-beta-(4-nitrophenyl)ethyl]acetamide | D-(-)-threo-2-Dichloroacetamido-1-(4-nitrophenyl)-1,3-propanediol | D-(-)-threo-2-Dichloroacetamido-1-p-nitrophenyl-1,3-propanediol |
| D-(-)-threo-Chloramphenicol | D-(-)-threo-N-Dichloroacetyl-1-p-nitrophenyl-2-amino-1,3-propanediol | D-(-)-threo-alpha, alpha-Dichloro-N-(beta-hydroxy-alpha-(hydroxymethyl)-p-nitrophenethyl)acetamide |
| D-Chloramphenicol | D-threo-(-)-2,2-Dichloro-N-(beta-hydroxy-alpha-(hydroxymethyl)-p-nitrophenethyl)acetamide | D-threo-(1R,2R)-1-p-Nitrophenyl-2-dichloroacetamido-1,3-propanediol |
| D-threo-1-(p-Nitrophenyl)-2-(dichloroacetylamino)-1,3-propanediol | D-threo-2,2-Dichloro-N-[beta-hydroxy-alpha-(hydroxymethyl)-4-nitrophenethyl]acetamide | D-threo-Chloramphenicol |
| D-threo-N-(1,1'-Dihydroxy-1-p-nitrophenylisopropyl)dichloroacetamide | D-threo-N-Dichloroacetyl-1-p-nitrophenyl-2-amino-1,3-propanediol | D-threo-N-dichloroacetyl-1-p-nitrophenyl-2-amino-1,3-propane-diol |
| D00104 | DB00446 | DSSTox_CID_265 |
| DSSTox_GSID_20265 | DSSTox_RID_75473 | DTXSID7020265 |
| Desphen | Detreomycin | Detreomycine |
| Dextromycetin | DivK1c_000544 | Doctamicina |
| Duphenicol | EINECS 200-287-4 | Econochlor |
| Econochlor (TN) | Elase-Chloromycetin | Elase-Chloromycetin (Salt/Mix) |
| Embacetin | Emetren | Enicol |
| Enteromycetin | Epitope ID:114066 | Erbaplast |
| Ertilen | F armicetina | FT-0602995 |
| Farmicetina | Fenicol | Globenicol |
| Glorous | Gloveticol | HMS2090M15 |
| HMS2095G03 | HMS2269N06 | HMS3712G03 |
| HMS501L06 | HSDB 3027 | HY-B0239 |
| Halcetin | Halomycetin | Hortfenicol |
| I 337A | IDI1_000544 | Interomycetine |
| Intramycetin | Intramyctin | Isicetin |
| Ismicetina | Isophenicol | Isopto fenicol |
| Juvamycetin | KBio1_000544 | KS-00000XTX |
| KS-000048O2 | Kamaver | Kemicetina |
| Kemicetine | Kloramfenikol | Klorita |
| Klorocid S | LS-225 | Laevomycetinum |
| Leukamycin | Leukomyan | Leukomycin |
| Levocin | Levomicetina | Levomitsetin |
| Levomycetin | Levoplast | Levosin |
| Levovetin | Loromicetina | Loromisan |
| Loromisin | M163 | MCULE-7778960570 |
| MFCD00078159 | MLS001055372 | MLS001066397 |
| MLS001332385 | MLS001332386 | MLS002222155 |
| Mastiphen | Mediamycetine | Medichol |
| Micloretin | Micochlorine | Micoclorina |
| Microcetina | Mychel | Mychel-Vet |
| Mycinol | Myclocin | Mycochlorin |
| Myscel | N-[(1R,2R)-2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl]-2,2-dichloroace tamide | NCGC00091011-01 |
| NCGC00091011-02 | NCGC00091011-03 | NCGC00091011-04 |
| NCGC00091011-05 | NCGC00091011-06 | NCGC00091011-08 |
| NCGC00091011-09 | NCGC00094620-01 | NCI-C55709 |
| NCI60_002620 | NINDS_000544 | NSC 16331 |
| NSC 3069 | NSC-3069 | NSC3069 |
| Normimycin V | Novochlorocap | Novomycetin |
| Novophenicol | Ocuphenicol | Oftalent |
| Oleomycetin | Opclor | Opelor |
| Ophthochlor | Ophthocort (Salt/Mix) | Ophtochlor |
| Optomycin | Otachron | Otophen |
| Pantovernil | Paraxin | Pentamycetin |
| Prestwick3_000031 | PubChem20321 | Q274515 |
| Quemicetina | RKL10087 | Rivomycin |
| Romphenil | Ronfenil | Ronphenil |
| SAM002589931 | SBB057728 | SC-18476 |
| SCHEMBL16111 | SMP1_000065 | SMR000471851 |
| SR-01000761450 | SR-01000761450-2 | SR-01000761450-3 |
| SR-01000761450-5 | ST024743 | SW198497-2 |
| Septicol | Sificetina | Sintomicetin |
| Sintomicetina | Sintomicetine R | Sno Phenicol |
| Sno-Phenicol | Soluthor | Stanomycetin |
| Synthomycetin | Synthomycetine | Synthomycine |
| Syntomycin | Tea-Cetin | Tega-Cetin |
| Tevcocin | Tevcosin | Thiamphenicol,(S) |
| Tifomycin | Tifomycine | Tiromycetin |
| Tox21_111306 | Tox21_400061 | Treomicetina |
| Tyfomycine | U-6062 | UNII-66974FR9Q1 |
| Unimycetin | Veticol | Vice ton |
| Viceton | W-2830 | WIIZWVCIJKGZOK-RKDXNWHRSA-N |
| WLN: WNR DYQY1QMVYGG | ZINC113382 | ZX-AFC000642 |
| chioramphenicol | chloramphenicol | s1677 |
| DrugBank Name | Chloramphenicol |
| DrugBank | DB00446 |
| CAS Number | 125440-98-4, 15318-45-3, 2787-09-9, 56-75-7, 7384-89-6, 85666-84-8 |
| PubChem Compound | 5959 |
| KEGG Compound ID | C00918 |
| KEGG Drug | D00104 |
| PubChem.Substance | 46505318 |
| ChEBI | 17698 |
| PharmGKB | PA448927 |
| ChemSpider | 5744 |
| BindingDB | 50028502.0 |
| TTD | DAP001356 |
| Wikipedia | Chloramphenicol |
| HET | CLM |
| DPD | 16112|8578 |