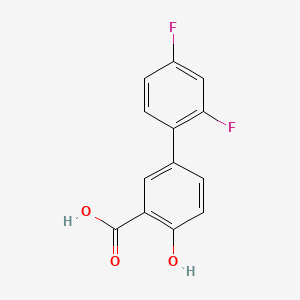
D0040 | Diflunisal
N
N02BA11 Diflunisal
[N02BA] Salicylic acid and derivatives
[N02B] OTHER ANALGESICS AND ANTIPYRETICS
[N02] ANALGESICS
[N] Nervous system
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| TRANSMEMBRANE POTENTIAL | 115 | 24hr | rat | hepatocytes | tetramethylrhodamine ethyl ester (TMRE) | decrease | AC50 (μM) | 40 |
| UNCOUPLING | increase | 40 | ||||||
| PROTONOPHORIC UNCOUPLING | 278 | |||||||
| MEMBRANE POTENTIAL | 17.9 µM | 30 mins | mouse | liver mitochondria | Rh123 fluorescence (excitation 485 nm, emission 535 nm) are recorded using a fluorescence multi-well plate reader (mCICCP (20 µM) treatments was considered as the 100% baseline for ΔΨm loss) | decrease | EC20 | 36 |
| RESPIRATION | 9.8 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | decrease | EC20 | 36 |
| RESPIRATION | ND | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | Negative | EC20 | 36 |
| STATE 2 RESPIRATION | 6.7 ± 1.3 | rat | isolated rat liver mitochondria | State 2 respiration ( 96-well plate format using a phosphorescent oxygen-sensitive probe MitoXpress) | inhibit | UC50 (nmol/mg mitochondrial protein) | 40 | |
| STATE 3 RESPIRATION | 100 nmol/mg mitochondrial protein | rat | isolated rat liver mitochondria | State 3 respiration ( 96-well plate format using a phosphorescent oxygen-sensitive probe MitoXpress) | Negative | IC50 (nmol/mg mitochondrial protein) | 40 | |
| OXYGEN CONSUMPTION RATE (OCR) | 15 μM | 2 minutes | human | HepG2 | Measurement of OCR | increase | EC50 | 7 |
| ECAR | 30 μM | 2 minutes | human | HepG2 | Measurement of ECAR | increase | EC50 | 7 |
| LIPID METABOLISM | 68 | 24hr | rat | hepatocytes | LipidTox, for neutral lipid accumulation, to evaluate lipid content. | accumulation | AC50 (μM) | 40 |
| GLUTATHIONE METABOLISM | 29 | 24hr | rat | hepatocytes | glutathion depletion: cells were incubated with 50 μM monochlorobimane with 6 μg/ml Hoechst 33342 | AC50 (μM) | 40 | |
| SWELLING | > 200 µM | 30 mins | mouse | liver mitochondria | swelling assay: Absorbance at 545 nm using a fluorescence multi-well plate reader (CaCl2 (50 µM) was considered as the 100% baseline for the swelling ) | increase | EC20 | 36 |
| ROS PRODUCTION | 558 | rat | hepatocytes | use CM-H2DCFDA to monitor reactive oxygen species | AC50 (μM) | 40 | ||
| CYTOCHROME C RELEASE | 79 | 24hr | rat | hepatocytes | cytochrome c release (anti-cytochrome c antibody ) | induce | AC50 (μM) | 40 |
| ER STRESS-INDUCED | 50 | 24hr | rat | hepatocytes | DNA damage 153 induction (GADD153 antibodies) for ER-stress induced apoptosis | induce | AC50 (μM) | 40 |
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| NADH:ubiquinone reductase | 9.8 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | inhibit | EC20 | 36 |
| Succinate dehydrogenase | ND | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | Negative | EC20 | 36 |
| Cytochrome c | > 200 µM | 30 mins | mouse | liver mitochondria | Cytochrome c release was evaluated using ELISA kit ( 20 µg/ml Alamethicin was used as 100% baseline) | release | EC20 | 36 |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Warning |
Aggregated GHS information provided by 39 companies from 2 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H302 (100%): Harmful if swallowed [Warning Acute toxicity, oral] H315 (97.44%): Causes skin irritation [Warning Skin corrosion/irritation] H319 (97.44%): Causes serious eye irritation [Warning Serious eye damage/eye irritation] H335 (97.44%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure Respiratory tract irritation] H361 (97.44%): Suspected of damaging fertility or the unborn child [Warning Reproductive toxicity] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P261, P264, P270, P271, P280, P281, P301+P312, P302+P352, P304+P340, P305+P351+P338, P308+P313, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| (1,1'-Biphenyl)-3-carboxylic acid, 2',4'-difluoro-4-hydroxy- | 1286107-99-0 | 1FL |
| 2',4'-Difluoro-4-hydroxy-(1',1-diphenyl)-3-carboxylic acid | 2',4'-Difluoro-4-hydroxy-(1,1'-biphenyl)-3-carboxylic acid | 2',4'-Difluoro-4-hydroxy-3-biphenylcarboxylic acid |
| 2',4'-Difluoro-4-hydroxy[1,1'-biphenyl]-3-carboxylic acid | 2',4'-difluoro-4-hydroxy-[1,1'-biphenyl]-3-carboxylic acid | 2',4'-difluoro-4-hydroxy-biphenyl-3-carboxylic acid |
| 2',4'-difluoro-4-hydroxybipheny-3-carboxylic acid | 2',4'-difluoro-4-hydroxybiphenyl-3-carboxylic acid | 2-(Hydroxy)-5-(2,4-difluorophenyl)benzoic acid |
| 22494-42-4 | 3-BIPHENYLCARBOXYLIC ACID, 2',4'-DIFLUORO-4-HYDROXY- | 5-(2,4-DIFLUOROPHENYL)-2-HYDROXY-BENZOIC ACID |
| 5-(2,4-Difluorophenyl)salicylic acid | 5-(2,4-difluorophenyl)-2-hydroxybenzoic acid | 5-[2,4-Difluorophenyl]salicylic acid |
| 5-[2,4-bis(fluoranyl)phenyl]-2-oxidanyl-benzoic acid | 7C546U4DEN | A816230 |
| AB00051969 | AB00051969-12 | AB00051969_13 |
| AB0013252 | AK161747 | AKOS005762917 |
| API0002353 | AX8003209 | Adomal |
| Algobid | BCP09905 | BDBM50240510 |
| BIDD:GT0063 | BPBio1_000151 | BRD-K22031190-001-05-3 |
| BRD-K22031190-001-13-7 | BRN 2654431 | BSPBio_000137 |
| BSPBio_002203 | C-15398 | C01691 |
| C13H8F2O3 | CAS-22494-42-4 | CC-26785 |
| CCG-40230 | CHEBI:39669 | CHEMBL898 |
| CPD000058723 | CS-0007468 | CTK1A3255 |
| Citidol | D00130 | DB-045934 |
| DB00861 | DSSTox_CID_2932 | DSSTox_GSID_22932 |
| DSSTox_RID_76792 | DTXSID5022932 | Difludol |
| Diflunisal (JAN/USP/INN) | Diflunisal [USAN:INN:BAN:JAN] | Diflunisal [USAN:USP:INN:BAN:JAN] |
| Diflunisal, European Pharmacopoeia (EP) Reference Standard | Diflunisal, United States Pharmacopeia (USP) Reference Standard | Diflunisal, analytical standard |
| Diflunisal-d3 | Diflunisalum | Diflunisalum [INN-Latin] |
| Diflusinal | DivK1c_000938 | Dolisal |
| Dolobid | Dolobid (TN) | Dolobil |
| Dolobis | EINECS 245-034-9 | FT-0630487 |
| Flovacil | Fluniget | Fluodonil |
| Flustar | GTPL7162 | H776 |
| HMS1568G19 | HMS1920G10 | HMS2090C16 |
| HMS2091M20 | HMS2095G19 | HMS3259G17 |
| HMS3712G19 | HMS502O20 | HUPFGZXOMWLGNK-UHFFFAOYSA-N |
| HY-18342 | IDI1_000938 | J-014739 |
| J10103 | KBio1_000938 | KBio2_001442 |
| KBio2_004010 | KBio2_006578 | KBio3_001423 |
| KBioGR_001085 | KBioSS_001442 | KS-00000KD6 |
| KS-1346 | LS-44258 | MCULE-8281018918 |
| MFCD00057834 | MK 647 | MK-647 |
| MLS000028678 | MLS001146895 | NC00506 |
| NCGC00016765-01 | NCGC00016765-02 | NCGC00016765-03 |
| NCGC00016765-04 | NCGC00016765-05 | NCGC00016765-06 |
| NCGC00016765-09 | NCGC00022783-03 | NCGC00022783-04 |
| NINDS_000938 | NSC-756728 | NSC756728 |
| Noaldol | Opera_ID_803 | PC11123 |
| Pharmakon1600-01500245 | Prestwick0_000039 | Prestwick1_000039 |
| Prestwick2_000039 | Prestwick3_000039 | Prestwick_168 |
| PubChem14748 | Q2602750 | Reuflos |
| SAM002554896 | SBB058143 | SBI-0051347.P003 |
| SC-13811 | SCHEMBL4337 | SEL14865206 |
| SMR000058723 | SPBio_001163 | SPBio_002058 |
| SPECTRUM1500245 | SR-01000003165 | SR-01000003165-2 |
| SR-01000003165-3 | ST24046572 | ST51014978 |
| Spectrum2_001012 | Spectrum3_000392 | Spectrum4_000513 |
| Spectrum5_000901 | Spectrum_000962 | TRA0051767 |
| Tox21_110598 | Tox21_110598_1 | UNII-7C546U4DEN |
| Unisal | ZINC20243 | ZX-AH027458 |
| ZX-AP008530 | [1,1'-Biphenyl]-3-carboxylic acid, 2',4'-difluoro-4-hydroxy- | [1,1'-Biphenyl]-3-carboxylicacid, 2',4'-difluoro-4-hydroxy- |
| diflunisal | s4609 |
| DrugBank Name | Diflunisal |
| DrugBank | DB00861 |
| CAS Number | 1286107-99-0, 22494-42-4, 27494-42-4 |
| PubChem Compound | 3059 |
| KEGG Compound ID | C01691 |
| KEGG Drug | D00130 |
| PubChem.Substance | 46507807 |
| ChEBI | 39669 |
| PharmGKB | PA449313 |
| ChemSpider | 2951 |
| BindingDB | 50240510.0 |
| TTD | DAP000152 |
| Wikipedia | Diflunisal |
| HET | 1FL |
| DPD | 2113 |