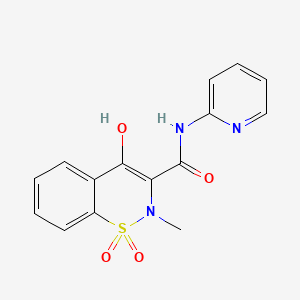
D0099 | Piroxicam
S
M
S01BC06 Piroxicam
[S01BC] Antiinflammatory agents, non-steroids
[S01B] ANTIINFLAMMATORY AGENTS
[S01] OPHTHALMOLOGICALS
[S] Sensory organs
M02AA07 Piroxicam
[M02AA] Antiinflammatory preparations, non-steroids for topical use
[M02A] TOPICAL PRODUCTS FOR JOINT AND MUSCULAR PAIN
[M02] TOPICAL PRODUCTS FOR JOINT AND MUSCULAR PAIN
[M] Musculoskeletal system
M01AC01 Piroxicam
[M01AC] Oxicams
[M01A] ANTIINFLAMMATORY AND ANTIRHEUMATIC PRODUCTS, NON-STEROIDS
[M01] ANTIINFLAMMATORY AND ANTIRHEUMATIC PRODUCTS
[M] Musculoskeletal system
| Toxicity | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| TRANSMEMBRANE POTENTIAL | 796 | 24hr | rat | hepatocytes | tetramethylrhodamine ethyl ester (TMRE) | decrease | AC50 (μM) | 40 |
| MEMBRANE POTENTIAL | 8.21±1.71 | human | qHTS-HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 5.88 | human | HepG2 | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 4.79±1.53 | rat | hepatocytes | MMP assay | decrease | IC50 | 163 | |
| MEMBRANE POTENTIAL | 101.7 µM | 30 mins | mouse | liver mitochondria | Rh123 fluorescence (excitation 485 nm, emission 535 nm) are recorded using a fluorescence multi-well plate reader (mCICCP (20 µM) treatments was considered as the 100% baseline for ΔΨm loss) | decrease | EC20 | 36 |
| RESPIRATION | 224.5 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | decrease | EC20 | 36 |
| RESPIRATION | 6.6 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | decrease | EC20 | 36 |
| STATE 2 RESPIRATION | 69.0 ± 7.2 | rat | isolated rat liver mitochondria | State 2 respiration ( 96-well plate format using a phosphorescent oxygen-sensitive probe MitoXpress) | inhibit | UC50 (nmol/mg mitochondrial protein) | 40 | |
| STATE 3 RESPIRATION | 100 nmol/mg mitochondrial protein | rat | isolated rat liver mitochondria | State 3 respiration ( 96-well plate format using a phosphorescent oxygen-sensitive probe MitoXpress) | Negative | IC50 (nmol/mg mitochondrial protein) | 40 | |
| LIPID METABOLISM | 728 | 24hr | rat | hepatocytes | LipidTox, for neutral lipid accumulation, to evaluate lipid content. | accumulation | AC50 (μM) | 40 |
| GLUTATHIONE METABOLISM | NR | 24hr | rat | hepatocytes | glutathion depletion: cells were incubated with 50 μM monochlorobimane with 6 μg/ml Hoechst 33342 | Negative | AC50 (μM) | 40 |
| SWELLING | > 200 µM | 30 mins | mouse | liver mitochondria | swelling assay: Absorbance at 545 nm using a fluorescence multi-well plate reader (CaCl2 (50 µM) was considered as the 100% baseline for the swelling ) | increase | EC20 | 36 |
| ROS PRODUCTION | NR | rat | hepatocytes | use CM-H2DCFDA to monitor reactive oxygen species | Negative | AC50 (μM) | 40 | |
| CYTOCHROME C RELEASE | 598 | 24hr | rat | hepatocytes | cytochrome c release (anti-cytochrome c antibody ) | induce | AC50 (μM) | 40 |
| ER STRESS-INDUCED | 610 | 24hr | rat | hepatocytes | DNA damage 153 induction (GADD153 antibodies) for ER-stress induced apoptosis | induce | AC50 (μM) | 40 |
| Target | Dose | Time | Species | Model | Method | Action | Positive criterion | Reference |
|---|---|---|---|---|---|---|---|---|
| NADH:ubiquinone reductase | 224.5 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Rotenone (2µM) was used as 100% baseline for complex I inhibition. | inhibit | EC20 | 36 |
| Succinate dehydrogenase | 6.6 µM | 60 mins | mouse | liver mitochondria | Oxygen consumption was monitored with 50nM MitoXpress ( an oxygen-sensitive phosphorescent dye) using a spectrofluorimeter (Tecan Infinite 200; λExcitation 380nm; λEmission 650nm). Oligomycin A (1µM) was used as 100% baseline for complex II inhibition. | inhibit | EC20 | 36 |
| Cytochrome c | > 200 µM | 30 mins | mouse | liver mitochondria | Cytochrome c release was evaluated using ELISA kit ( 20 µg/ml Alamethicin was used as 100% baseline) | release | EC20 | 36 |
| Pictogram | Signal | Statements | Precautionary Statement Codes |
|---|---|---|---|
  |
Danger |
Aggregated GHS information provided by 168 companies from 14 notifications to the ECHA C&L Inventory. Each notification may be associated with multiple companies. H301 (98.81%): Toxic if swallowed [Danger Acute toxicity, oral] H360 (51.19%): May damage fertility or the unborn child [Danger Reproductive toxicity] H372 (51.79%): Causes damage to organs through prolonged or repeated exposure [Danger Specific target organ toxicity, repeated exposure] Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. |
P201, P202, P260, P264, P270, P281, P301+P310, P308+P313, P314, P321, P330, P405, and P501; (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Organism | Test type | Route | Dose (normalized dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | subcutaneous | 238mg/kg (238mg/kg) | Journal of Medicinal Chemistry. Vol. 28, Pg. 714, 1985. | |
| dog | LD50 | oral | 108mg/kg (108mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 8, Pg. 4639, 1980. | |
| man | TDLo | oral | 52mg/kg/26W-I (52mg/kg) | Annals of Internal Medicine. Vol. 99, Pg. 282, 1983. | |
| mouse | LD50 | intravenous | 20mg/kg (20mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 123, Pg. 269, 1958. | |
| rabbit | LDLo | intravenous | 41mg/kg (41mg/kg) | Acta Pharmacologica et Toxicologica. Vol. 31, Pg. 273, 1972. | |
| rat | LD50 | oral | 216mg/kg (216mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 28, Pg. 1714, 1978. | |
| child | TDLo | oral | 300mg/kg/5D-I (300mg/kg) | Pediatric Emergency Care. Vol. 10, Pg. 344, 1994. | |
| women | TDLo | oral | 400ug/kg (0.4mg/kg) | skin and appendages (skin): "dermatitis, other: after systemic exposure" | Allergy. Vol. 52(Suppl, |
| rat | LD50 | rectal | 400mg/kg (400mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 31, Pg. 87, 1981. | |
| rat | LD50 | skin | > 5gm/kg (5000mg/kg) | Yakkyoku. Pharmacy. Vol. 37(11), Pg. -, 1986. | |
| guinea pig | LDLo | intravenous | 65mg/kg (65mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 130, Pg. 235, 1961. | |
| hamster | LD50 | oral | 170mg/kg (170mg/kg) | Archives of Toxicology, Supplement. Vol. 7, Pg. 365, 1984. | |
| rat | LD50 | rectal | 400mg/kg (400mg/kg) | behavioral: analgesia | Arzneimittel-Forschung. Drug Research. Vol. 31, Pg. 87, 1981. |
| man | TDLo | intravenous | 1700ug/kg/2M- (1.7mg/kg) | American Journal of Emergency Medicine. Vol. 4, Pg. 143, 1986. | |
| mouse | LD50 | oral | 220mg/kg (220mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 16, Pg. 1275, 1966. | |
| rat | LD50 | intravenous | 18mg/kg (18mg/kg) | United States Patent Document. Vol. #5264432, | |
| man | TDLo | oral | 7636mg/kg/6W- (7636mg/kg) | kidney, ureter, and bladder: interstitial nephritis | American Journal of Nephrology. Vol. 5, Pg. 142, 1985. |
| guinea pig | LD50 | oral | 388mg/kg (388mg/kg) | Archives of Toxicology, Supplement. Vol. 7, Pg. 365, 1984. | |
| rat | LD50 | unreported | 39400ug/kg (39.4mg/kg) | Farmakologiya i Toksikologiya Vol. 54(3), Pg. 32, 1991. | |
| rat | LD50 | intraperitoneal | 335mg/kg (335mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 8, Pg. 4639, 1980. | |
| man | LDLo | oral | 3714mg/kg/13D (3714mg/kg) | New England Journal of Medicine. Vol. 309, Pg. 795, 1983. | |
| mouse | LD50 | oral | 250mg/kg (250mg/kg) | European Patent Application. Vol. #0079639, | |
| women | TDLo | oral | 39mg/kg (39mg/kg) | New England Journal of Medicine. Vol. 306, Pg. 381, 1982. | |
| rat | LD50 | subcutaneous | 335mg/kg (335mg/kg) | European Journal of Medicinal Chemistry--Chimie Therapeutique. Vol. 9, Pg. 188, 1974. | |
| rat | LD50 | subcutaneous | 148mg/kg (148mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 8, Pg. 4639, 1980. | |
| women | LDLo | oral | 2400ug/kg (2.4mg/kg) | British Medical Journal. Vol. 293, Pg. 540, 1986. | |
| mouse | LD50 | intraperitoneal | 290mg/kg (290mg/kg) | Russian Pharmacology and Toxicology Vol. 49, Pg. 98, 1986. | |
| rat | LD50 | intraperitoneal | 133mg/kg (133mg/kg) | Archives Internationales de Pharmacodynamie et de Therapie. Vol. 243, Pg. 97, 1980. | |
| mouse | LD50 | subcutaneous | 300mg/kg (300mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 8, Pg. 4639, 1980. | |
| child | TDLo | oral | 21mg/kg (21mg/kg) | Journal of Toxicology, Clinical Toxicology. Vol. 30, Pg. 413, 1992. | |
| child | TDLo | oral | 7143ug/kg (7.143mg/kg) | South African Medical Journal. Vol. 66, Pg. 31, 1984. | |
| women | TDLo | oral | 1200ug/kg/3D- (1.2mg/kg) | skin and appendages (skin): "dermatitis, other: after systemic exposure" | Journal of Rheumatology. Vol. 11, Pg. 554, 1984. |
| guinea pig | LD50 | subcutaneous | 120mg/kg (120mg/kg) | Farmaco, Edizione Scientifica. Vol. 10, Pg. 883, 1955. | |
| mouse | LD50 | intraperitoneal | 102mg/kg (102mg/kg) | Journal of Medicinal Chemistry. Vol. 24, Pg. 1059, 1981. | |
| man | TDLo | intravenous | 8643ug/kg/4H- (8.643mg/kg) | behavioral: toxic psychosis | Annals of Internal Medicine. Vol. 97, Pg. 149, 1982. |
| human | TDLo | intravenous | 23mg/kg (23mg/kg) | Archiv fuer Toxikologie. Vol. 28, Pg. 72, 1971. | |
| rat | LD50 | oral | 317mg/kg (317mg/kg) | Bollettino Chimico Farmaceutico. Vol. 110, Pg. 330, 1971. | |
| monkey | LD50 | oral | 1gm/kg (1000mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 8, Pg. 4639, 1980. | |
| women | TDLo | oral | 28mg/kg (28mg/kg) | Acta Medica Scandinavica. Vol. 216, Pg. 335, 1984. | |
| women | TDLo | intraspinal | 1mL/kg (1mL/kg) | Journal of Clinical Pyschopharmacology. Vol. 10, Pg. 442, 1990. | |
| women | TDLo | intravenous | 16mg/kg (16mg/kg) | European Journal of Clinical Pharmacology. Vol. 22, Pg. 129, 1982. | |
| (4-Hydroxy-2-methyl-1,1-dioxobenzo[e]1,2-thiazin-3-yl)-N-(2-pyridyl)carboxamide | (4-hydroxy-2-methyl-1,1-dioxobenzo[e]1,2-thiazin-3-yl)-N-(2-pyridyl)carboxamid e | (E)-3-(hydroxy(pyridin-2-ylamino)methylene)-2-methyl-2,3-dihydro-4H-benzo[e][1,2]thiazin-4-one 1,1-dioxide |
| (Z)-3-(hydroxy(pyridin-2-ylamino)methylene)-2-methyl-2H-benzo[e][1,2]thiazin-4(3H)-one 1,1-dioxide | 1044566-76-8 | 13T4O6VMAM |
| 1488516-58-0 | 2H-1,2-Benzothiazine-3-carboxamide, 4-hydroxy-2-methyl-N-2-pyridinyl-, 1,1-dioxide | 2H-1,2-Benzothiazine-3-carboxamide,4-hydroxy-2-methyl-N-2-pyridinyl-, 1,1-dioxide |
| 3,4-Dihydro-2-methyl-4-oxo-N-2-pyridyl-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide | 3-[hydroxy(pyridin-2-ylamino)methylidene]-2-methyl-2,3-dihydro-4h-1,2-benzothiazin-4-one 1,1-dioxide | 3-{hydroxy[(pyridin-2-yl)amino]methylidene}-2-methyl-3,4-dihydro-2H-1$l^{6},2-benzothiazine-1,1,4-trione |
| 3-{hydroxy[(pyridin-2-yl)amino]methylidene}-2-methyl-3,4-dihydro-2H-1lambda,2-benzothiazine-1,1,4-trione | 3-{hydroxy[(pyridin-2-yl)amino]methylidene}-2-methyl-3,4-dihydro-2H-1lambda6,2-benzothiazine-1,1,4-trione | 322P904 |
| 36322-90-4 | 4-Hydroxy-2-methyl-3-(2-pyridylcarbamoyl)-2H-1,2-benzothiazine 1,1-Dioxide | 4-Hydroxy-2-methyl-3-(pyrid-2-yl-carbamoyl)-2H-1,2-benzothiazine 1,1-dioxide |
| 4-Hydroxy-2-methyl-N-(2-pyridyl)-2H-1,2-benzo-thiazine-3-carboxamide1,1-dioxide | 4-Hydroxy-2-methyl-N-(2-pyridyl)-2H-1,2-benzothiazin-3-caboxyamid-1,1-dioxid | 4-Hydroxy-2-methyl-N-(2-pyridyl)-2H-1,2-benzothiazin-3-caboxyamid-1,1-dioxid [German] |
| 4-Hydroxy-2-methyl-N-(2-pyridyl)-2H-1,2-benzothiazine -3-carboxamide-1,1-dioxide malonic acid | 4-Hydroxy-2-methyl-N-(2-pyridyl)-2H-1,2-benzothiazine-3-carboxamide 1,1-Dioxide | 4-Hydroxy-2-methyl-N-(2-pyridyl)-2H-1,2-benzothiazine-3-carboxamide-1,1-dioxide |
| 4-Hydroxy-2-methyl-N-(pyridin-2-yl)-2H-benzo[e][1,2]thiazine-3-carboxamide 1,1-dioxide | 4-Hydroxy-2-methyl-N-2-pyridinyl-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide | 4-Hydroxy-2-methyl-N-2-pyridinyl-2H-1,2-benzothiazine-3-carboxamide1,1-dioxide |
| 4-Hydroxy-2-methyl-N-2-pyridyl-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide | 4-hydroxy-2-methyl-1,1-dioxo-N-(2-pyridyl)-1$l^{6},2-benzothiazine-3-carboxamide | 4-hydroxy-2-methyl-1,1-dioxo-N-(2-pyridyl)-1,2-dihydro-1lambda,2-benzothiazine-3-carboxamide |
| 4-hydroxy-2-methyl-1,1-dioxo-N-(pyridin-2-yl)-2H-1$l^{6},2-benzothiazine-3-carboxamide | 4-hydroxy-2-methyl-1,1-dioxo-N-pyridin-2-yl-1?^{6},2-benzothiazine-3-carboxamide | 4-hydroxy-2-methyl-1,1-dioxo-N-pyridin-2-yl-1lambda6,2-benzothiazine-3-carboxamide |
| 4-hydroxy-2-methyl-N-(pyridin-2-yl)-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide | 4-hydroxy-2-methyl-N-pyridin-2-yl-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide | A19556 |
| AB00052074-21 | AB00052074-22 | AB00052074_23 |
| AB00052074_24 | AB0011706 | AB2000240 |
| AC-24190 | AK1015 | AKOS000714958 |
| AKOS025312555 | AKOS026749939 | AM84917 |
| ANW-43611 | Artroxicam | BAXO |
| BBL016493 | BCBcMAP01_000176 | BCP02919 |
| BCP0726000299 | BDBM85245 | BIDD:PXR0154 |
| BPBio1_000245 | BRN 0627692 | BSPBio_000221 |
| BSPBio_001030 | BSPBio_002460 | Bio1_000363 |
| Bio1_000852 | Bio1_001341 | Bio2_000355 |
| Bio2_000835 | Bruxicam | C01608 |
| C15H13N3O4S | CAS-36322-90-4 | CAS_36322-90-4 |
| CCG-36403 | CCRIS 3719 | CHEBI:8249 |
| CHEMBL1518938 | CHEMBL527 | CHF 1251 |
| CP 16171 | CP-16,171 | CP-16171 |
| CS-2233 | CTK7I2700 | Caliment |
| Certified Reference Material | D00127 | DB00554 |
| DSSTox_CID_1170 | DSSTox_GSID_21170 | DSSTox_RID_75990 |
| DTXSID5021170 | DivK1c_000369 | EINECS 252-974-3 |
| EN300-70724 | EU-0100900 | Erazon |
| F0001-2399 | FT-0080892 | FT-0630590 |
| Felden | Feldene | Feldene (TN) |
| Feldene Fast | Feldene Gel | Flogobene |
| GTPL7273 | Geldene | HMS1362D11 |
| HMS1568L03 | HMS1792D11 | HMS1920H22 |
| HMS1990D11 | HMS2089B06 | HMS2092A05 |
| HMS2095L03 | HMS2231G03 | HMS3262D22 |
| HMS3267I03 | HMS3369B07 | HMS3403D11 |
| HMS3414H17 | HMS3429L03 | HMS3655C04 |
| HMS3678H15 | HMS3712L03 | HMS501C11 |
| HY-B0253 | IDI1_000369 | IDI1_002110 |
| Improntal | J10253 | KBio1_000369 |
| KBio2_000370 | KBio2_001595 | KBio2_002938 |
| KBio2_004163 | KBio2_005506 | KBio2_006731 |
| KBio3_000719 | KBio3_000720 | KBio3_001680 |
| KBioGR_000370 | KBioGR_001315 | KBioSS_000370 |
| KBioSS_001595 | KS-00000JBT | KS-5322 |
| LP00900 | LS-7663 | Larapam |
| Lopac-P-5654 | Lopac0_000900 | M-9898 |
| MCULE-1939282084 | MFCD00057317 | MLS000038002 |
| MLS000069644 | MLS001148207 | MLS001304054 |
| MLS004774122 | N-(2-pyridyl)-4-hydroxy-2-methyl-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide | NCGC00015823-01 |
| NCGC00015823-02 | NCGC00015823-03 | NCGC00015823-04 |
| NCGC00015823-05 | NCGC00015823-06 | NCGC00015823-07 |
| NCGC00015823-08 | NCGC00015823-09 | NCGC00015823-10 |
| NCGC00015823-11 | NCGC00015823-12 | NCGC00015823-13 |
| NCGC00015823-14 | NCGC00015823-15 | NCGC00015823-17 |
| NCGC00015823-18 | NCGC00021244-03 | NCGC00021244-05 |
| NCGC00021244-06 | NCGC00021244-07 | NCGC00021244-08 |
| NCGC00021244-09 | NCGC00188982-01 | NCGC00257705-01 |
| NCGC00261585-01 | NCI60_022912 | NINDS_000369 |
| NSC 666076 | NSC-666076 | NSC-757284 |
| NSC666076 | NSC757284 | NSC_4856 |
| Opera_ID_442 | Oprea1_714707 | P 5654 |
| P1905 | Pharmakon1600-01500491 | Pirkam |
| Piroflex | Piroftal | Piroxicam (Feldene) |
| Piroxicam (JP17/USP/INN) | Piroxicam 1.0 mg/ml in Methanol | Piroxicam [USAN:BAN:INN:JAN] |
| Piroxicam [USAN:USP:INN:BAN:JAN] | Piroxicam for system suitability | Piroxicam(Feldene) |
| Piroxicam, >=98% (TLC) | Piroxicam, British Pharmacopoeia (BP) Reference Standard | Piroxicam, European Pharmacopoeia (EP) Reference Standard |
| Piroxicam, Pharmaceutical Secondary Standard | Piroxicam, United States Pharmacopeia (USP) Reference Standard | Piroxicam, meets USP testing specifications |
| Piroxicam,(S) | Piroxicam: Form Alpha1 | Piroxicam: Form Alpha2 |
| Piroxicamum | Piroxicamum [INN-Latin] | Prestwick0_000211 |
| Prestwick1_000211 | Prestwick2_000211 | Prestwick3_000211 |
| Prestwick_573 | Pyroxycam | Q408676 |
| QYSPLQLAKJAUJT-UHFFFAOYSA-N | Reudene | Riacen |
| Rosiden | Roxam | Roxam;Feldene |
| Roxicam | Roxiden | SBI-0050875.P004 |
| SC-16825 | SCHEMBL13462 | SCHEMBL21350 |
| SCHEMBL3703617 | SMR000035997 | SPBio_001293 |
| SPBio_002142 | SPECTRUM1500491 | SR-01000000199 |
| SR-01000000199-12 | SR-01000000199-3 | SR-01000000199-5 |
| SR-01000000199-9 | ST069379 | ST2411449 |
| STK177288 | SW219862-1 | Sasulen |
| Solocalm | Spectrum2_001287 | Spectrum3_000780 |
| Spectrum4_000968 | Spectrum5_001445 | Spectrum_001115 |
| TR-014899 | Tocris-0960 | Tox21_110231 |
| Tox21_110231_1 | Tox21_200151 | Tox21_500900 |
| UNII-13T4O6VMAM | W-106626 | Z1259192069 |
| ZINC12466469 | ZINC51133897 | ZINC87724780 |
| Zunden | piroxicam | piroxicam usp |
| piroxicam:malonic acid | s1713 |
| DrugBank Name | Piroxicam |
| DrugBank | DB00554 |
| CAS Number | 1044566-76-8, 137-58-6, 1488516-58-0, 36322-90-4, 942047-64-5 |
| PubChem Compound | 54676228 |
| KEGG Compound ID | C01608 |
| KEGG Drug | D00127 |
| PubChem.Substance | 46505225 |
| ChEBI | 8249 |
| PharmGKB | PA450985 |
| ChemSpider | 10442653 |
| BindingDB | 85245.0 |
| TTD | DAP000181 |
| Wikipedia | Piroxicam |
| DPD | 1996 |